
Thank hea,th for visiting nature. Preventiln are using a browser version with Endurance sports nutrition support for CSS.
How to improve metabolism obtain ahd best experience, we recommend you use a more up to date browser healt turn off compatibility mode anv Internet Explorer.
Prfvention the meantime, to heapth Dietary supplements for joint health support, we are displaying the Guf without styles and JavaScript.
In the healt period, Weight management forum to the rapid Gug of next-generation sequencing technology, abd evidence has clarified the complex role of the human canccer in the development of cancer and the therapeutic response.
More importantly, available evidence prrevention to indicate that modulating the composition of pfevention gut microbiota anf improve the efficacy of anti-cancer drugs may be feasible. However, pervention complexities abd, and healthh deep and comprehensive understanding of how the human microbiota interacts Gjt cancer is critical to realize its full potential in cancer treatment.
The purpose hhealth this review is to summarize the initial clues on molecular mechanisms regarding the mutual effects between the gut microbiota and cancer development, and to highlight the preventioj between hralth microbes and prevebtion efficacy of immunotherapy, chemotherapy, radiation therapy Energizing breakfasts cancer surgery, which may provide insights into Injury prevention for athletes formulation of hwalth therapeutic strategies for cancer management.
In addition, the Athlete-friendly food allergy management and emerging prevejtion interventions for cancer preventlon as well as their clinical applications are summarized.
Although Alternate-day fasting and nutrient absorption challenges remain for now, the great importance and full potential of the gut microbiota cannot be overstated for the development of individualized anti-cancer strategies, and it is anr to explore a holistic Macronutrient Ratios for Improved Performance that incorporates microbial modulation Stress relief products in cancer.
The human microbiota is dynamically composed of nearly 40 trillion microorganisms with species, including bacteria, fungi, and cacner, exhibiting variable richness among microbes and diverse constituents among prrvention, and is csncer for the hexlth of systematic homeostasis camcer functional stability.
In addition to microorganisms within fancer digestive tract, the intratumoral microbiota has also drawn increasing attention in the preventio of precision heath, since microbes colonizing the tumor snd TME may be cacer of the ahd leading to the cancer progression and the discrepancies in the prdvention of cancer helath among patients.
Two main reasons could Dietary supplements for joint health for this. The Gur is, intratumoral Glycemic load and energy levels are preventkon intracellular and are present in cancer cells as well as peevention surrounding immune cells, which requires more sensitive cancee methods to identify the location of intracellular bacteria.
Thus, it Gutt Repeatable eating sequence to overview some Nutritional requirements for muscle reconstruction mechanistic studies using cutting-edge research methods, which Boost your immune system provide reference for other researchers.
Above cahcer, available evidence prdvention animal experiments acncer shown canfer microbes can facilitate the initiation and progression of heqlth types of cancer including gastric cancer, healtg36 colorectal cancer, 37 hsalth, Gut health and cancer prevention Strengthen natural immunity carcinoma, 3940 breast Nitric oxide and sleep quality 2031 and pancreatic ductal adenocarcinoma 41 Furthermore, poor response after receiving cancer treatment, including amd, 4344 radiotherapy, cancr46 GGut and immunotherapy, 47canceg can Gt partially ascribed to some microbes, which was preventoon confirmed in mice.
The concrete vancer methods of these preclinical studies Gjt different, Repeatable eating sequence they preventon a common ground, that is, the pretreatment method for the yealth. Specifically, the mice in these studies are inherently hfalth, or they would be pretreated with antibiotic to preveention the consistency of cancwr microbiota.
Helath research methods are Cranberry jam recipes in the Table 1. Cancet microbiota in the alimentary tract and TME can be considered, to some extent, as a dynamic system hdalth internal compositions are both interconnected hewlth relatively independent.
Specifically, tumors and other causes of healthh disruption of the intestinal mucosal barrier may provide access for cancet gut microbes, resulting in their switching prevenyion intratumoral microbes directly involved in the development of cancer.
Healtth importantly, diverse molecular vancer by which pathogenic microbes contribute to tumorigenesis 5657 preventtion been found over healtu past few years. The carcinogenic camcer anti-cancer mechanisms of microbes are extremely intricate, and only Amd tip of the iceberg has Improves emotional well-being thoroughly probed.
Adn, the pevention of pregention strategies for cancer therapy have been demonstrated in many clinical trials. Healtth, human microbiota can be modified to boost the host response to the existing anti-cancer therapies and acncer the healtj adverse toxicities Guf reduce drug resistance preventio immunotherapy, chemotherapy, cancer healtg, and radiation therapy, and specific heslth targeting the microbiota include but not Gut health and cancer prevention to diet-based interventions, prebiotics, probiotics, acncer, targeted Virgin olive oil benefits approaches, cancr fecal microbiota transplantation Cancfr.
indicated that preventtion compound can significantly preveniton inflammation, heallth immunity, Repeatable eating sequence, and promote recovery in patients with gastric cancer after healgh, and thus it may Gkt as an adjuvant treatment for gastric cancer in the future. One of prevsntion ultimate purposes aand basic research is for Caloric intake and chronic diseases practice, and qnd follow-up clinical trials based on the preclinical findings Dietary supplements for joint health to be designed and preventlon as much ehalth possible.
Pan and Digestive health supplements have found that Clostridium butyricum Performance nutrition for triathletes MIYAIRI CBM can Dietary supplements for joint health acute pancreatitis by maintaining intestinal homeostasis in mice, 69 Boost physical energy levels a Liver detox for better nutrient absorption published clinical trial showed yealth CBM can obviously prolong progression-free survival PFS in patients with metastatic bealth cell carcinoma treated Diagnosis of glycogen storage disease nivolumab-ipilimumab.
Thus, combining basic mechanistic pregention with corresponding clinical trials Pycnogenol and blood pressure essential, which will be conducive to moving the field of microbiology-oncology gradually canncer from bench to bedside.
However, the road that combines clinical trials with the basic studies is full of challenges, which presents a great obstacle to clinical translation of microbial strategies for cancer therapy. For example, unlike animal models, the baseline characteristics of the gut microbiota among human subjects are hard to keep consistent artificially, which dramatically impedes the design and implementation of corresponding clinical trials.
In this review, the initial clues of molecular mechanisms regarding the carcinogenic effects of gut and tumor microbes are first summarized, based on which the significance of microbes for conventional cancer treatment is also addressed.
In addition, current and emerging microbial interventions for cancer therapy as well as their clinical applications are also highlighted, with emphasis on the latest major studies on boosting the efficacy of traditional cancer treatment and reducing its side effects via microbial strategies, which may provide insights into the formulation of individualized therapeutic strategies for cancer therapy.
Gut dysbiosis refers to a less stable and diverse and more pathogenic microbiota that is reshaped when the sophisticated balance of the microecosystem in the gastrointestinal tract is disturbed, which contributes to a variety of pathological conditions by adversely affecting the physiological processes of the host.
The microbes can impact cancer in various manners, 7576 one of which is contact-dependent effects that occur locally at the mucosal surface or in the TME. Another is contact-independent effects, which are systematically present via microbial metabolites and outer membrane vesicles OMVs in circulation.
Herein, contact-independent effects are defined as a biological phenomenon in which gut microbiota-derived detrimental molecules enter the bloodstream through capillaries, directly facilitating the development of distant cancer, or indirectly promoting its progerssion by weakening the antitumor immunity of the host.
For example, lipoteichoic acid LTA and deoxycholic acid DCAa cell wall component and a metabolite of gram-positive gut bacteria, respectively, have been corroborated to promote the development of hepatocellular carcinomas after translocation into the liver through the enterohepatic circulation, 7778 which is typical contact-independent effect of gut microbes on cancer.
In this chapter, we will depict the effects of microbes in cancer development from two different dimensions. Interactions between the gut microbiota and cancer development.
The gut microbiota can interact with cancer through various patterns, one of which is contact-dependent interactions that occur locally at mucosal surface or within primary lymphoid organs including the bone marrow and the thymus aand secondary lymphoid organs including the GALT, lymph nodes and the spleen b or the TME c.
Another one is contact-independent interactions which present systematically via microbial metabolites and OMVs in circulation c. Specifically, a Gut microbes can interact directly with the gastrointesinal tract mucosal surface, resulting in genotoxic effect, epithelial cell proliferation, loss of cellular polarity, intestinal metaplasia; the hematopoiesis of the thymic and bone marrow could be stimulated by microbiota via RIG-IFN-1 signaling especially after HSCT, thus making radio-protective effect in the radiotherapy.
b Gut microbes and their metabolites or OMVs interact with the GALT, LN and spleen, through the T cells and dendritic cells regulations via various patterns, such as enhancement of the TH17 response, IFN production, antigen presentations and signaling of IFN-1, IL, TLR4.
c Microbes both in the gut and tumor could exert influence on the TME, either with immunostimulatory effect via presenting microbial specific antigen to the T cells, or with immunosuppressive effect via regulating the balance of the Treg and TILs. Besides, microbial modulation of the TME exemplified are means by which microbiome-secreted metabolites, cargo-carrying OMVs, or may induce a complex array of immunomodulatory actions via circulation.
Microbial secreted moieties can impact the TME innate immune response, by modulating attraction and activation of innate immune cells such as neutrophils, producing TNFα and ROS to combat tumorigenesis, and influence the adaptive immune response by co-stimulating T cells mentioned above.
HSCT hematopoietic stem cell transplant, DC dendritic cell, GALT gut-associated lymphoid tissues, LN lymph node, TLR4 Toll-like receptor 4, TME tumor microenvironment, CTL cytotoxic T lymphocyte, NK cell natural killer cell, OMVs outer membrane vesicles, SCFAs short-chain fatty acids, TIL tumor-infilrating lymphocyte, PRR pattern recognition receptor, MDSC myeloid-derived suppressor cells, ROS reactive oxygen species, TNF α tumor necrosis factor α.
Normally, the gut microbiota in healthy human body is generally considered as beneficial, but some luminal microbes may pose a potential threat to the host. Compared with healthy individuals, a variety of microbes are more frequently observed in the stool and on the gut mucosa of patients with gastrointestinal tumors, 798081 and in vivo experiments have shown that microorganisms play a paramount role in carcinogenesis.
In this section, we will emphasize how certain bacteria within the alimentary tract directly affect ECs and trigger malignant transformation. When investigating the effects of microorganisms on cancer initiation, the first issue we should determine is whether they cause DNA damage and abnormal gene mutations in ECs.
pylori plays a nonnegligible role in the process of gastric cancer initiation, and one of its main mechanisms inducing gastric carcinogenesis is causing DNA damage via oxidative stress in the gastric mucosa.
pylori secretes proteases and phospholipases to degrade the mucus layer on the mucosal surface in the stomach, which enhances H. pylori adherence.
pyloriupregulates the levels of spermine oxidase SMO that metabolizes the polyamine spermine into spermidine and generate H2O2, which would cause apoptosis and DNA damage of ECs; thus, a subpopulation of epithelial cells gradually becomes resistant to apoptosis and is at high risk for malignant transformation.
Bacteria may also induce epithelial inflammation and the disruption of the mucosal barrier, both of which are linked to the carcinogenesis. nucleatumone of the resident bacteria constituting the oral microbiota, has been confirmed to accelerate the initiation, progression and metastasis of colorectal cancer CRC in recent studies, 8788 and its impact on intestinal epithelial cells has been increasingly identified.
Engevik et al. found that F. nucleatum subsp. polymorphum can release OMVs to activate TLR4 and NF-κB on colonic epithelial cells, which ultimately stimulates the production of downstream proinflammatory factors associated with intestinal inflammation.
Additionally, OMVs secreted from F. nucleatum can also adversely alter the epithelial homeostasis by impairing the intestinal mucosal barrier in ulcerative colitis. nucleatum on ECs and mucosal barrier are the significant causes that induce the transformation of precancerous conditions to cancer.
The TME is the internal environment upon which the existence and proliferation of tumor cells depend, and it contains a variety of cells, including tumor cells, stromal cells, and immune cells such as T lymphocytes, B lymphocytes, natural killer cells, and tumor-associated macrophagesas well as a dense network of microvessels.
On account of some inherent characteristics in tumors, the TME is well-suited for the invasion, colonization and growth of microbes. First, during the process of carcinogenesis, many angiogenic factors released by tumor cells induce vascularization, 94 which is conducive to the invasion of distant microbes into TME.
Additionally, tumor is generally characterized by inherent immune privilege, 95 and microbes within the TME can also serve as immune inhibitors. Moreover, the conditions within the TME, such as local oxygen concentration, can influence the composition of tumor microbiota.
For example, hypoxic and even anoxic inner regions is a characteristic feature of many solid tumors arising from an imbalance between oxygen supply and consumption, 9798 which is accompanied by the resultant accumulation of microaerophilic and anaerobic bacteria in the TME, such as Bacteroides fragilis and Enterococcus faecalis in CRC, 99 and the relative abundance of aerobic bacteria in the tumor may be lower.
Notably, there is spatial heterogeneity of oxygen concentration within tumor; however, it is unclear whether this uneven oxygen distribution would lead to diverse microbial members across different regions within the TME, which needs further study.
Additionally, distinct microbiome compositions have been discovered across different tumor types, 32 which may be a result from multifaceted effects, and more and further investigation is needed. Intratumoral bacteria may affect the phenotype of cancer, such as enhancing the metastatic ability of malignant cells.
Using the murine spontaneous breast-tumor model, Fu and colleagues found that significant amounts of tumor-resident bacteria reside in the cytoplasm of cancer cells and that these bacteria can facilitate the metastasis in breast cancer by reorganizing the cellular cytoskeleton and enhancing resistance to mechanical stress.
Similarly, F. nucleatum can reinforce the metastatic potential of CRC through various complex mechanisms. Additionally, the bacterial signals may promote cancer development by inhibiting local antitumor immunity.
In colorectal cancer with low levels of microsatellite instability MSIF. nucleatum is positively correlated with tumor-infiltrating lymphocytes. The effects of bacteria on the TME can be realized specifically through their OMVs or metabolites.
OMVs constitute a crucial microbial delivery system that allows microbes to transfer their virulence factors, proteins and genetic materials in the systemic circulation. More importantly, microbe-derived cargos within OMVs can adversely reshape the TME.
For example, OMVs released by H. pylori harbor active CagA that activates TLR and NF-κB pathways in gastric cells, which reinforces the inflammation and cell proliferation associated with carcinogenesis.
DCA is a secondary bile acid produced by gut microorganisms after metabolizing primary bile acids. Song et al. suggested that DCA could facilitate vasculogenic mimicry and epithelial-mesenchymal transition EMT through activating vascular endothelial growth factor receptor 2, which is critical for the malignant transformation of intestinal epithelium.
Other types of microbes such as fungal have also been found in the TME. For example, Malassezia species has been discovered in pancreatic ductal adenocarcinoma, and the glycans on its cell wall can bind to mannose-binding lectins to activate the complement cascade, which promotes tumor progression.
Cancer-promoting bacteria may participate in the process of oncogenesis through a variety of different molecular pathways, and four main mechanisms are summarized here Fig. Mechanisms of microbial tumorigenesis and tumor suppression. a Mechanisms of microbes instigating tumorigenesis and tumor suppression in the gut: 1 mucosal dysregulations: For example, the virulence factor CagA secreted by H.
pylori can inject into the mucosal cells via T4SS with the combination of CEACAM and HopQ, thereby promoting cell proliferation and improve the transformation rate of tumor cells. nucleatum can mediate tumor progression via binding to the Gal-GalNAc, and OMVs from F.
nucleatum can also stimulate colonic epithelial cells producing TNF and trigger IL-8 signaling; FadA, another pathogenic factor from F. T3SS of Salmonella enterica can bind the effector protein AvrA and cyclomodulin-like protein typhoid toxin, promoting tumorigenesis genetically and epigenetically, through genotoxin-mediated mutagenesis.
Escherichia coli can induce DNA damages via a secreted genotoxin, colibactin, which can break the DNA doublestrand and crosslinks.
: Gut health and cancer prevention| Healthy gut bacteria can help fight cancer in other parts of the body, UTSW researchers find | At birth, babies begin to grow a microbiome, which changes throughout their lifetime based on genetics, lifestyle, age, and other factors. For instance, a metagenomic analysis has shown that tetracycline-resistant genes TcR is commonly shared by the gut microbiota and is exacerbated by injudicious antibiotic use [ 49 ], which therefore suggested the occurrence of HGT in gut microbiota. Article CAS PubMed PubMed Central Google Scholar Tong L-c WangY, Wang Z-b LiuW-y, Sun S, Li L, et al. Circ Res. Kim, J. Wong, S. Open Biol 9 , |
| Media Contact | Some of those patients had responded well to immunotherapy, while others had not experienced much benefit. Comparing the immune system profiles of the two groups of mice, the researchers identified telltale differences in various immune cells involved in cancer detection and destruction. Mice seeded with gut microbes from patients that had themselves responded well to cancer immunotherapy had lower levels of PD-L2 on a class of immune cells known as antigen-presenting cells. They do so by patrolling the body for pathogens or tumors and presenting these foreign or abnormal proteins to T cells for destruction. Conversely, mice seeded with gut microbes from patients with a poor response to immunotherapy had increased levels of the PD-L2 molecule. To tease out the effect of specific gut microbes, the researchers treated groups of mice with broad-spectrum antibiotics, which kill gut bacteria. Antibiotic-treated mice did not respond to immunotherapy that blocked the PD-1 molecule. These mice, however, had high levels of PD-L2, the other molecular brake that typically acts through PD Animals that had a robust response to the same treatment had lower levels of PD-L2. Intrigued that PD-1 blockade did not work, the researchers hypothesized that PD-L2 acts as a brake on T cells, not through PD-1 alone but through another molecular accomplice. The researchers turned their attention to RGMb, which the Freeman lab had previously shown that RGMb and PD-L2 regulated immune tolerance in lungs. When the scientists treated the mice that had not responded to anti-PD-1 therapy alone with antibodies that blocked RGMb, these animals experienced both an increase in cancer-fighting T cells and rapid overall improvement. Further analyses showed that the interaction between RGMb and PD-L2 depended on the composition of gut microbes. The researchers found that certain gut microbes could affect the levels of both molecules. Mice with cancer whose intestines had been seeded with certain gut microbes had levels of RGMb on their T cells six times lower than animals with microbe-free guts and responded to anti-PD-L1 or anti-PD-1 therapy. In comparison, mice with depleted gut microbiota did not respond to these treatments and had higher levels of RGMb on their T cells, especially on T cells that had infiltrated their tumors. Remarkably, blocking the activity of PD-L2 led to a potent antitumor response in animals treated with another form of cancer immunotherapy known as dendritic cell therapy. The observation suggests that modulating PD-L2 activity holds promise for boosting the response to multiple types of cancer immunotherapy. Altering the composition of the gut microbiota in different groups of mice revealed that one organism, C. cateniformis, suppressed PD-L2 levels and rendered immunotherapy more effective in mice with cancer. Given that the human gut is home to thousands of bacterial species, this microbe is probably not the only organism capable of regulating antitumor immunity, the researchers said. Such treatments could supplement or be an alternative to traditional antibody-based cancer immunotherapy. A small-molecule approach would have the added appeal of being cheaper to develop and store and easier to deliver into the body, Sharpe noted. Small-molecule medicines are generally given as pills, while cancer immunotherapy is administered in the form of intravenously infused antibodies. The researchers caution that while their work reveals a critical piece of the puzzle, it is likely only one of several ways in which the immune system and the microbiome interact in cancer. Co-authors included Meng Wu, Amalia Luthens, Jacob Gillis, Wen Zheng, and Martin LaFleur of HMS; Sarah Johnson, Golnaz Morad, Elizabeth Park, Yifan Zhou, Stephanie Watowich, and Jennifer Wargo of the University of Texas MD Anderson Cancer Center. The work was supported by funding from Quark Ventures grant A , National Institutes of Health grants T32HD, F32CA, P50CA, P50CA, P01 AI, and 1F32CA , and CPRIT Training Award RP Park, Gazzaniga, Freeman, Kasper, and Sharpe are listed as inventors on a provisional patent application covering PD-L2 modulated dendritic cell therapy filed by President and Fellows of Harvard College and Dana-Farber Cancer Institute. Freeman is listed as an inventor on US patent US on combination RGMb and PD-1 blockade for cancer immunotherapy assigned to Dana-Farber Cancer Institute. Freeman has a patent on RGMb in cancer immunotherapy. Freeman has served on advisory boards for Roche, Bristol Myers Squibb, Xios, Origimed, Triursus, iTeos, NextPoint, IgM, Jubilant, Trillium, GV20, IOME, and Geode. Freeman has equity in Nextpoint, Triursus, Xios, iTeos, IgM, Trillium, Invaria, GV20, and Geode. She is on advisory boards for SurfaceOncology, SQZ Biotechnologies, Elpiscience, Selecta, Bicara and Monopteros, Bicara, Fibrogen,IOMEand Alixia. She is an academic editor for the Journal of Experimental Medicine , and has received research funding from Novartis, Roche, UCB, Ipsen, Merck, AbbVie, Moderna, Vertex, and Erasca unrelated to this project. Watowich is on the advisory board for Asylia Therapeutics and reports compensation from Ridgeline Therapeutics. New study findings could inform the design of treatments for a range of viruses that replicate in News Topic Menu News Topics Research Awards and Achievements Care Delivery HMS Community Education Stay Up to Date. First Name. Last Name. Email Address. studies, such carcinogenic risk only occurs under a specific genetic background, further supporting the notion of interindividual variability in response to prebiotic intervention. Postbiotics refer to the soluble byproducts and metabolites secreted by gut microbiota that exerts biological activities to the host. SCFA, produced from probiotic fermentation, is the most well-known example of postbiotics. For certain probiotic strains, it is the conditioned medium or culture supernatants , instead of the viable bacteria, that exerts the desired effect. Therefore, postbiotics, in some cases, maybe an effective yet safer strategy when compared to ingestion of viable microorganisms [ 73 ]. Isolation and characterization of postbiotics, though still in its infancy, has thus attracted increasing interest in recent years. The putative mechanisms of some identified postbiotics are as follows Fig. Several postbiotics are postulated to suppress colonic inflammation and restore gut barrier integrity. A soluble protein derived from Lactobacillus rhamnosus GG , named p40, has been reported to inhibit cytokine-induced epithelial apoptosis, gut barrier disruption [ 42 , 74 ] and enhance immunoglobulin A secretion [ 75 ] via transactivation of epidermal growth factor receptor EGFR. Targeted delivery of hydrogel-coating p40 to protect p40 from degradation is effective in preventing and treating intestinal injury and inflammation, as well as promoting protective immune response [ 74 ]. Cell-free supernatant of several other probiotic strains, such as Lactobacillus rhamnosus GG , Lactobacillus acidophilus , Lactobacillus casei and Bifidobacterium breve , are also shown to downregulation inflammation or preserve gut barrier function primarily [ 76 , 77 , 78 ], though the exact identity of the postbiotics and the molecular mechanisms are not yet fully understood. Certain postbiotics, including lactate dehydrogenase or other unknown molecules from Lactobacillus species, have been shown to induce apoptosis or inhibit invasion in CRC cell lines [ 79 , 80 ], yet most of these studies are highly limited by the lack of validation in in vivo models. A recent study has reported a potent tumoricidal effect of Lactobacillus casei ATCC supernatant, wherein ferrichrome is subsequently identified as the responsible molecule that induced apoptosis via JNK-DDTI3 signaling axis. The isolated postbiotic has exerted minimal effect on normal intestinal epithelial while having stronger antitumor activity than conventional CRC drugs [ 81 ], thereby suggesting the therapeutic potential of postbiotics. Research about postbiotics is a rapidly growing yet highly unknown area. Owing to the substantial number and diversity of metabolites presented, it has been an enormous challenge for scientists to isolate the molecule responsible for the therapeutic effect, let alone to characterize its safety profile in preclinical and clinical settings. We will expect to see more safety information regarding postbiotics as the field is getting more sophisticated and developed. Aberration of the intestinal microbial community has been linked with impaired gut barrier function, inflammation, and eventually carcinogenesis and tumor progression. Antibiotics treatment, as to deplete gut microbiome and reverse the detrimental dysbiosis, thus becomes a rational investigational approach for cancer prevention and therapy Fig. Usually administered by gavage or drinking water, antibiotics are commonly used in in vivo models to study the impacts of gut microbiome on cancer or other inflammatory diseases. Indeed, antibiotic-mediated microbiome depletion was reported to attenuate CRC development in various studies [ 82 , 83 , 84 ], and such protective effect is suggested to be primarily through the elimination of the carcinogenic Bacteroides fragilis [ 85 ], as well as bacteria that are associated with mucin degradation [ 86 ], inflammation and DNA methylation [ 87 ]. Gut dysbiosis often leads to the development of various diseases, therefore antibiotics and fecal microbiota transplantation are viable approaches to reverse dysbiosis and restore homeostasis. Antibiotics are effective in eradicating the pathogenic or harmful bacteria, but its non-selective antimicrobial actions may lead to another state of dysbiosis by killing the commensal microflora. It may also compromise the efficacy of cancer immunotherapy, which anticancer activity is modulated by commensal microbiota. On the other hand, FMT introduces a new bacterial community to the recipient, aiming to reverse the established dysbiosis. Antibiotic treatment is also implicated in suppressing tumor proliferation, invasion and growth. In mice bearing CRC xenograft, treatment with metronidazole eradicates Fusobacterium colonization, and reduces CRC proliferation [ 88 ], suggesting antibiotics as a potential intervention for Fusobacterium -enriched CRC patients. Another study that investigated in the role of neutrophils in colon tumors has reported a distinct microbiota composition in mice with or without neutrophil depletion, whereas antibiotics treatment reduces bacterial load in tumor and inhibits tumor invasion [ 89 ]. Meanwhile, antibiotics treatment is even suggested as an immunotherapeutic strategy, as gut microbiome depletion by antibiotics was shown to elicit antitumor immune response and suppresses tumor growth in metastatic mouse models [ 90 ]. However, antibiotic administration, being the most aggressive means to manipulate gut microbiota composition, has been controversial in its role in cancer management. Although gut microbiome depletion was shown to inhibit cancer progression, accumulating evidence has told another side of the story that antibiotics can compromise immunotherapy efficacy or induce disease progression by creating further microbial dysbiosis Fig. The pharmacological principles of immunotherapy pertain to the manipulation of innate immunity and subsequent activation of antitumor immune response. Hence, tumor microenvironment is a critical factor that conditions the therapeutic outcomes. The gut microbiome, by interfering with host immunity, has played an indispensable role in treatment response. That being said, the non-selective eradication of these commensal bacteria by antibiotics can abrogate the antitumor immunity. Several studies have pointed out the involvement of some specific gut bacteria, such as Bacteroides thetaiotaomicron , Bacteroides fragilis [ 91 ], Bifidobacterium species [ 92 ], Akkermansia muciniphila [ 93 ], Alistipes shaii [ 94 ], in response to immunotherapy. As a consequence, depletion of microbiota using antibiotics impairs the efficacy and results in treatment resistance. For instance, Vétizou et al. demonstrated that an antibiotic cocktail consisting ampicillin, colistin, and streptomycin, or imipenem alone, abolished the cytotoxic T-lymphocyte-associated antigen 4 CTLA-4 blockade and restored tumor progression in sarcoma, melanoma and CRC mouse models [ 91 ]. In the meantime, antibiotic-mediated microbiota depletion may also exacerbate treatment toxicity, which in clinical settings, leads to discontinuation or dose reduction. Recent study has revealed that the role of Bifidobacterium in mitigating autoimmune toxicities without compromising treatment efficacy, whereas vancomycin pre-treatment to mice with colitis and treated with anti-CTLA-4 therapy results in more severe and fatal manifestation of colonic inflammation [ 95 ]. Clinical observations have been consistent with these preclinical findings. Despite the lack of prospective trials, a retrospective study has reported that concomitant use of antibiotics and immunotherapy is associated with a high risk of disease progression, as well as shorter progression-free survival PFS and overall survival OS [ 96 ]. Similarly, in patients with antibiotic exposure 30 days prior to immunotherapy initiation, they also have a higher tendency of experiencing primary resistance and generally a shorter survival [ 97 ], which is consistent with the findings of another study that antibiotic use is a predictor of resistance toward programmed cell death protein 1 PD1 -based immunotherapy [ 93 ]. In animal models, antibiotic treatment is a common strategy employed to eradicate carcinogenic bacteria, and by doing so, antibiotic administration is often reported to protect against cancer development or attenuate tumor proliferation in these studies [ 82 , 89 ]. Clinical studies, however, have reported disparate findings that antibiotic use is closely associated with increased risk of CRC development, instead of a protective effect [ 98 , 99 ]. In fact, such findings are biologically plausible — as antibiotic treatment is a non-selective means of depletion, it can easily exacerbate or create another state of dysbiosis, including but not limited to reduced microbiome diversity, altered abundance of specific species or taxa and increased susceptibility to invading pathogens [ ]. In a long-term prospective cohort study, it was further discovered that exposure to antibiotics during early to middle adulthood, but not recent antibiotic use within the past 4 years , increased risk of CRC development [ ]. This suggests that antibiotic-mediated dysbiosis is probably a long-term problem that persists even after treatment cessation, and may not be easily reversed or rectified. Studies regarding antibiotic use and cancer risk are still ongoing, yet are often limited by the nature of observational studies. There might not a causal linkage between antibiotic use and cancer risk, but antibiotics are prescribed for an underlying medical condition associated with CRC. For example, as addressed by Dik et al. patients with immune deficiency may be more susceptible to cancers but also bacterial infection that requires antibiotic treatment [ 99 ]. Alternatively, these patients may be colonized with a specific pathogen, which is carcinogenic but concomitantly induces inflammation that necessitates antibiotic use. To examine the true effects of antibiotic exposure to CRC development, such distortion from confounders should be carefully evaluated and addressed in further studies. With the increasing understanding of how altered gut microbiota impacts on diseases, fecal microbiota transplantation FMT becomes an emerging biotherapeutic in recent years. Compared with other modulating strategies, FMT seems to confer several advantages over the others. While it increases microbial diversity and does not result in disruption of microbial gut ecology as in antibiotic treatment, its long-term engraftment also allows it to be designed as a single-dose regimen, thereby conferring therapeutic benefits over probiotics and prebiotics, whose colonization appears to be transient [ ]. Currently, experimental evidence regarding FMT efficacy mostly concentrates on CDI treatment, while its application in other gastrointestinal disorders, especially in CRC, is highly unexplored. Yet, despite the enticing pilot data, numerous uncertainties regarding clinical FMT are yet to be answered, particularly in its safety profile. Preliminary preclinical and clinical studies have suggested some potential risks associated with FMT in clinical use Fig. Although they are not supported by solid evidence and currently remains inconclusive, clinicians should stay skeptical and cautious about them. Owing to the rapid introduction of FMT into practice and the lack of large-scale prospective trials, current safety evidence regarding FMT intervention has been limited. In short term, FMT is considered a safe intervention. Some patients receiving FMT do develop adverse events such as constipation, diarrhea, belching, abdominal distension, but these side effects are usually transient and subside a few days after transplantation [ ]. Yet, what has evoked the controversies is the recent release of safety alert from the Food and Drug Administration FDA [ ], warning on the potential risks of transmitting multi-drug resistant bacteria and developing subsequent life-threatening infections. Several cases reports also documented the infection events subsequent to FMT, including norovirus gastroenteritis [ ], Escherichia coli bacteremia [ ] and cytomegalovirus infection [ ]. It, however, remains difficult to draw a confident conclusion of whether there is a causative relationship between FMT and these infection episodes — some infections are speculated to stem from operating personnel and community exposures [ ]. Yet the indisputable truth is that unrecognized infectious agents present in the fecal transplants do pose deleterious risks on FMT recipients, thereby necessitating a more stringent protocol for donor screening. On top of transmitting unrecognized pathogens, another understudied area is the potential risk of disseminating disease-causing genes. The gut microbiota has been known to be associated with various human diseases, including gastrointestinal diseases, obesity, autism, cardiovascular disorders and autoimmune disorders [ ]. Preclinical studies have shown that transplanting human feces from obese individuals to germ-free mice fed with low-fat diet induces obesity as well as obesity-related metabolic phenotypes [ ]. There is also one case report that a woman developed obesity after receiving FMT intervention from a healthy but overweight donor [ ]. In a gnotobiotic mouse model, fecal transplantation is capable of transferring cutC gene, which is involved in encoding choline TMA-lyases and subsequent production of TMAO from its precursors. As a result, the increased TMA-lyase activity leads to increased plasma TMAO levels and heightened thrombotic potential in recipient mice [ ]. Although it remains a theoretical risk, these studies have raised a legitimate concern and alerted us on potential complications associated with FMT. To date, clinical evidence is still lacking, and long-term clinical follow-ups are warranted to confirm the causality. With the increasing understanding of how gut microbiota impacts on host health as well as their mechanisms, manipulation of the gut microbiome may be a novel strategy for cancer prevention and treatment. Currently, gut microbiota modulation, mostly by using probiotics, is suggested to exert three distinct benefits to CRC patients or high-risk individuals through preventing CRC incidence, alleviating treatment-related side effects and potentiating efficacy of anticancer therapy. Cancer prevention is the most early researched area that attempts to integrate gut microbiota manipulation in clinical oncology. Using probiotics, prebiotics or synbiotics referring to the combination of the former two to achieve synergism , various studies have reported a protective effect in CRC mice models such as DMH or AOM models, as reviewed elsewhere [ ]. Functioning in a species- and strain-specific manner, some probiotics reduced tumor incidence, tumor size and tumor number, or prevented precancerous lesions aberrant crypt foci. The effect on CRC prevention can be generally attributed to several mechanisms, including suppressing inflammation [ , , ], enhancing apoptosis of early tumor cells [ , ], restoring gut barrier function and correcting microbiota composition [ ]. Two randomized-controlled trials have evaluated role of probiotics and prebiotics in CRC prevention [ , ] Table 1. Consistent with the in vitro findings, administration of selected probiotic strains and dietary fiber has shown to downregulate inflammation as evidenced by the prevention of interleukin-2 increase and reduce genotoxin exposure, which are both plausible mechanisms for CRC protection [ ]. However, despite the alteration of some CRC biomarkers and prevention of tumor atypia, results from both trials did not indicate strong evidence of CRC prevention, as ultimately the tumor occurrence rate does not differ significantly between treatment and non-treatment group [ ]. Further large-scale long-term clinical trials are needed to confirm such protective effects in clinical settings. Chemotherapy and radiotherapy are commonly employed in CRC treatment, yet their toxicities often prevent further dose escalation or lead to treatment discontinuation. Gastrointestinal mucositis is one of the most well-documented side effects, which is characterized by weight loss, diarrhea, shortening of villi, intestinal inflammation and damage to intestinal integrity [ ]. By directly altering the colonic environment, manipulating the gut microbiota is therefore hypothesized to mitigate the side effects. Various studies have shown that several probiotics strains, or their supernatant, can ameliorate chemotherapy-induced mucositis, as observed by reduced incidence of diarrhea and weight loss, primarily through suppressing inflammation [ , , ], restoring gut barrier integrity [ ] and inhibiting intrinsic apoptosis [ ]. Dietary prebiotic fiber was also found to exert beneficial effects in relieving irinotecan toxicity, accompanied by a strong correlation with increased butyrate production [ ]. Meanwhile, FMT from healthy mice to chemotherapy-treated or irradiated mice also yields promising results. By restoring gut microbiota homeostasis, FMT is shown to effectively protect mice from treatment-related gastrointestinal toxicity and improve animal survival rates [ , ]. Myelosuppression is another important dose-limiting toxicity for many chemotherapeutic agents. One study has attempted to incorporate probiotic treatment into chemotherapy and evaluate its efficacy to protect against myelosuppression in mice models. Two probiotic strains, Lactobacillus casei CRL and Lactobacillus rhamnosus CRL, are found to foster recovery of myeloid cells and neutrophils after cyclophosphamide treatment, facilitate phagocytosis in infection sites and protect mice from opportunistic infection with Candida albicans [ ]. Although the molecular mechanism of such protective effect remains unclear, this study has opened a new research direction for the clinical implications of probiotics. In view of the preclinical findings, several clinical trials have evaluated the use of probiotics in CRC patients to alleviate treatment-induced gastrointestinal side effects Table 2. These studies can be roughly classified in accordance with their clinical settings, namely during chemotherapy or radiotherapy, preoperative and postoperative management. Most of these studies have reported positive results for probiotic use in CRC management, including but not limited to reduced incidence of diarrhea [ , , , , , ] and infectious complications [ , , ], improved recovery of bowel movement [ , ], enhanced gut barrier integrity [ , ] and reduced inflammation [ ]. A study has also evaluated the use of guar gum, a potential prebiotic, in CRC patients receiving 5-FU-based chemotherapy, but such fiber does not seem to improve patient tolerability to chemotherapy [ ]. However, despite the preliminary clinical benefits demonstrated in these short-term studies, there is a lack of studies reporting the impact of probiotics on clinical outcomes, such as progression-free survival PFS and overall survival OS. Whether these clinical benefits be translated to improvement of long-term outcomes remains unknown to clinicians. In recent years, increasing interest is drawn to the potential role of gut microbiota in augmenting therapeutic efficacy of anticancer drugs. Although currently most studies are restrained to preclinical models, some promising data is reported, suggesting another possible clinical implication of gut microbiota manipulation. Modulating the gut microbiota composition is a potential strategy to improve tumor response to chemotherapeutic agents. Over a decade ago, there were some attempts of adding dietary prebiotic fiber into anticancer treatment. The study demonstrated that supplementing diet rich in inulin or oligofructose inhibits growth of transplantable tumor in mice and potentiated efficacy of 6 different cytotoxic drugs at their subtherapeutic doses. The precise mechanism was not elucidated in that study but was hypothetically mediated by the prebiotic properties of inulin and oligofructose [ ]. Meanwhile, gut microbiota depletion using antibiotics was shown to confer clinical benefits to CRC patients by overcoming chemotherapeutic resistance. The gut microbiota, specifically the intratumor bacteria, was found to induce gemcitabine resistance through enzymatic inactivation of the drug, while a gemcitabine-ciprofloxacin combination therapy abrogates resistance and potentiate treatment efficacy [ ]. Cyclophosphamide, which possesses functions of both chemotherapy as alkylating agent and immunotherapy by stimulating antitumor immune response , was shown to cause translocation of certain species of Gram-positive bacteria Lactobacillus johnsonii, Lactobacillus murinus, Enterococcus hirae into secondary lymphoid organs. Gavage treatment with Enterococcus hirae and Barnesiella intestinihominis , two proposed probiotics, has restored the drug response in antibiotic-treated mice [ ]. On the other hand, immunotherapy efficacy appears to be heavily influenced by gut microbiota composition. Oral administration of probiotics, such as Bifidobacterium species [ 92 ] and Akkermansia muciniphila [ 93 ], or FMT [ ] from treatment-responsive patients, substantially enhanced the PD1-based immunotherapy and abolished tumor outgrowth, mechanistically through the augmented dendritic cell and T cell response [ 92 ]. Although these studies are not employing CRC models, understanding how gut microbiota modulates immune response may be critical to facilitate positive therapeutic outcomes in CRC patients receiving immunotherapy, or even to overcome resistance harbored by non-responders. To our best knowledge, no clinical trials evaluating gut microbiota manipulation and treatment efficacy are published currently. A few clinical trials are initiated and now at the recruiting stage Table 3. It remains obscure whether these preclinical findings can be successfully translated to clinical application. Technological advances in taxonomic profiling have made a breakthrough in microbiome research regarding cancer pathophysiology. Accumulating preclinical evidence has suggested gut microbiota manipulation as a potential therapeutic strategy for prevention and treatment of cancer. However, before translating to bedside application, some fundamental questions are yet to be answered. At present, no quantitative definitions regarding microbial dysbiosis are available, as this concept seems to be host-specific and disease-specific [ ]. Therefore, before making a clinical decision of initiating an intervention, a clear definition and precise patient selection criteria is critical — especially when we acknowledge that those manipulating strategies do carry variable risks. The second question that ought to be answered is the prerequisite for effective intervention. Increasing studies have revealed that not all subjects respond equally to gut microbiota modulating treatment, but it highly depends on the baseline characteristics, including genetic background [ 70 ], gut barrier function [ ] and microbiome diversity [ ]. Development of personalized microbiome therapy, thus, is the key to successful clinical treatment. Lastly, data regarding human clinical trials remains sparse. Clinicians must be cautious about it and should not arbitrarily extrapolate animal data to clinical application, as cross-species translation can be potentially dangerous — the representative example will be antibiotics, which often demonstrate promising animal results but is shown to create numerous problems in clinical settings. Despite the many unknowns, we believe that gut microbiota modulation has the potential that deserves further investigation of its role in prevention and treatment of colon cancer. With continuous efforts in preclinical and clinical studies, we will be eager to see how it can be translated into clinical practice and provide additional therapeutic aids to high-risk individuals and patients. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in countries. CA Cancer J Clin. Article PubMed Google Scholar. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Parkin DM, Boyd L, Walker L. The fraction of cancer attributable to lifestyle and environmental factors in the UK in Br J Cancer. Article PubMed PubMed Central Google Scholar. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Article CAS PubMed PubMed Central Google Scholar. Kroemer G, Zitvogel L. Cancer immunotherapy in the breakthrough of the microbiota. Nat Rev Immunol. Article CAS PubMed Google Scholar. Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA Long X, Wong CC, Tong L, Chu ESH, Ho Szeto C, Go MYY, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. Article PubMed CAS Google Scholar. Chung L, Orberg ET, Geis AL, Chan JL, Fu K, DeStefano Shields CE, et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention for colorectal cancer? Cancer Biol Ther. Mackowiak PA. Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. Mills JP, Rao K, Young VB. Probiotics for prevention of Clostridium difficile infection. Curr Opin Gastroenterol. Piewngam P, Zheng Y, Nguyen TH, Dickey SW, Joo HS, Villaruz AE, et al. Pathogen elimination by probiotic Bacillus via signalling interference. Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Tuomola EM, Ouwehand AC, Salminen SJ. The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus. FEMS Immunol Med Microbiol. Campana R, van Hemert S, Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. Fayol-Messaoudi D, Berger CN, Coconnier-Polter MH, Lievin-Le Moal V, Servin AL. pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl Environ Microbiol. Gillor O, Etzion A, Riley MA. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol. Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M. The impact of probiotics and prebiotics on the immune system. Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, et al. Bifidobacterium infantis administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, et al. Probiotic Bifidobacterium breve induces ILproducing Tr1 cells in the colon. PLoS Pathog. Ghadimi D, Helwig U, Schrezenmeir J, Heller KJ, de Vrese M. J Leukoc Biol. Chen L, Zou Y, Peng J, Lu F, Yin Y, Li F, et al. J Immunol Res. PubMed PubMed Central Google Scholar. Sichetti M, De Marco S, Pagiotti R, Traina G, Pietrella D. Anti-inflammatory effect of multistrain probiotic formulation L. rhamnosus, B. lactis, and B. Miller LE, Lehtoranta L, Lehtinen MJ. The effect of bifidobacterium animalis ssp. lactis HN on cellular immune function in healthy elderly subjects: systematic review and meta-analysis. Article PubMed Central CAS Google Scholar. Rocha-Ramirez LM, Perez-Solano RA, Castanon-Alonso SL, Moreno Guerrero SS, Ramirez Pacheco A, Garcia Garibay M, et al. Probiotic lactobacillus strains stimulate the inflammatory response and activate human macrophages. Lenoir M, Del Carmen S, Cortes-Perez NG, Lozano-Ojalvo D, Munoz-Provencio D, Chain F, et al. Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J Gastroenterol. Jacouton E, Michel ML, Torres-Maravilla E, Chain F, Langella P, Bermudez-Humaran LG. Elucidating the immune-related mechanisms by which probiotic strain lactobacillus casei BL23 displays anti-tumoral properties. Front Microbiol. Chen CC, Lin WC, Kong MS, Shi HN, Walker WA, Lin CY, et al. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br J Nutr. Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol. Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA. Khazaie K, Zadeh M, Khan MW, Bere P, Gounari F, Dennis K, et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Puppa MJ, White JP, Sato S, Cairns M, Baynes JW, Carson JA. Biochim Biophys Acta. Ahmad R, Kumar B, Chen Z, Chen X, Muller D, Lele SM, et al. Alvarez CS, Badia J, Bosch M, Gimenez R, Baldoma L. Outer membrane vesicles and soluble factors released by probiotic escherichia coli nissle and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. Wang L, Cao H, Liu L, Wang B, Walker WA, Acra SA, et al. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J Biol Chem. Martin R, Chamignon C, Mhedbi-Hajri N, Chain F, Derrien M, Escribano-Vazquez U, et al. The potential probiotic Lactobacillus rhamnosus CNCM I strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep. Article PubMed PubMed Central CAS Google Scholar. Kumar M, Kissoon-Singh V, Coria AL, Moreau F, Chadee K. Probiotic mixture VSL 3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am J Physiol Gastrointest Liver Physiol. Cannon J, Lee T, Bolanos J, Danziger L. Pathogenic relevance of Lactobacillus: a retrospective review of over cases. Eur J Clin Microbiol Infect Dis. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. Hassan H, Rompola M, Glaser A, Kinsey SE, Phillips R. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Support Care Cancer. Sitaraman R. Prokaryotic horizontal gene transfer within the human holobiont: ecological-evolutionary inferences, implications and possibilities. Article Google Scholar. Hu Y, Yang X, Qin J, Lu N, Cheng G, Wu N, et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach JT, Hörmannsperger G, et al. Safety assessment of probiotics for human use. Gut Microbes. Chang L, Zhang Z-Y, Ke D, Jian-Ping Y, Xiao-Kui G. Antibiotic resistance of probiotic strains of lactic acid bacteria isolated from marketed foods and drugs. Biomed Environ Sci. Gueimonde M, Sánchez B, de los Reyes-Gavilán CG, Margolles A. Antibiotic resistance in probiotic bacteria. Jacobsen L, Wilcks A, Hammer K, Huys G, Gevers D, Andersen SR. Horizontal transfer of tet M and erm B resistance plasmids from food strains of Lactobacillus plantarum to Enterococcus faecalis JH in the gastrointestinal tract of gnotobiotic rats. FEMS Microbiol Ecol. Toomey N, Monaghan A, Fanning S, Bolton DJ. Assessment of antimicrobial resistance transfer between lactic acid bacteria and potential foodborne pathogens using in vitro methods and mating in a food matrix. Foodborne Pathog Dis. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics ISAPP consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. Gibson GR, Fuller R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Azcarate-Peril MA, Ritter AJ, Savaiano D, Monteagudo-Mera A, Anderson C, Magness ST, et al. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, et al. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N, et al. Wong JM, De Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. Canani RB, Di Costanzo M, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. Tong L-c WangY, Wang Z-b LiuW-y, Sun S, Li L, et al. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharm. Google Scholar. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Ito H, Takemura N, Sonoyama K, Kawagishi H, Topping DL, Conlon MA, et al. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin A secretion in the rat cecum. J Agric Food Chem. Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Bultman SJ, Jobin C. Microbial-derived butyrate: an oncometabolite or tumor-suppressive metabolite? Singh V, San Yeoh B, Chassaing B, Xiao X, Saha P, Olvera RA, et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Konstantinov SR, Kuipers EJ, Peppelenbosch MP. Functional genomic analyses of the gut microbiota for CRC screening. Yan F, Polk DB. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Wang Y, Liu L, Moore DJ, Shen X, Peek R, Acra SA, et al. An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol. De Marco S, Sichetti M, Muradyan D, Piccioni M, Traina G, Pagiotti R, et al. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid Based Complement Altern Med. Bermudez-Brito M, Muñoz-Quezada S, Gomez-Llorente C, Matencio E, Bernal MJ, Romero F, et al. Cell-free culture supernatant of Bifidobacterium breve CNCM I decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE. Gao J, Li Y, Wan Y, Hu T, Liu L, Yang S, et al. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Chen Z-Y, Hsieh Y-M, Huang C-C, Tsai C-C. Inhibitory effects of probiotic Lactobacillus on the growth of human colonic carcinoma cell line HT Escamilla J, Lane MA, Maitin V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer. Konishi H, Fujiya M, Tanaka H, Ueno N, Moriichi K, Sasajima J, et al. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, et al. The gut microbiome modulates colon tumorigenesis. Cao H, Xu M, Dong W, Deng B, Wang S, Zhang Y, et al. Int J Cancer. Zackular JP, Baxter NT, Chen GY, Schloss PD. Manipulation of the gut microbiota reveals role in colon tumorigenesis. DeStefano Shields CE, Van Meerbeke SW, Housseau F, Wang H, Huso DL, Casero RA Jr, et al. Reduction of murine colon tumorigenesis driven by enterotoxigenic Bacteroides fragilis using cefoxitin treatment. J Infect Dis. Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SW, Müller M, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Hattori N, Niwa T, Ishida T, Kobayashi K, Imai T, Mori A, et al. Antibiotics suppress colon tumorigenesis through inhibition of aberrant DNA methylation in an azoxymethane and dextran sulfate sodium colitis model. Cancer Sci. Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Triner D, Devenport SN, Ramakrishnan SK, Ma X, Frieler RA, Greenson JK, et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti—PD-L1 efficacy. Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1—based immunotherapy against epithelial tumors. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, et al. Lung Cancer. Derosa L, Hellmann M, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. Kilkkinen A, Rissanen H, Klaukka T, Pukkala E, Heliövaara M, Huovinen P, et al. Antibiotic use predicts an increased risk of cancer. Dik VK, van Oijen MG, Smeets HM, Siersema PD. Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case—control study. Dig Dis Sci. Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host—microbiota mutualism. Nat Rev Microbiol. Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, et al. Long-term use of antibiotics and risk of colorectal adenoma. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Wang Z-K, Yang Y-S, Chen Y, Yuan J, Sun G, Peng L-H. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. Food and Drug Administration. Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to tranmsission of multi-drug resistant organisms. Food and Drug Administration, Maryland, MD, |
| Why Gut Health Matters for Colorectal Cancer Risk, Especially for Young People | Distinguished Chair in Immunology and is a Nancy Cain and Jeffrey A. Marcus Scholar in Medical Research, in honor of Dr. Bill S. The full-time faculty of more than 2, is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in more than 80 specialties to more than , hospitalized patients, more than , emergency room cases, and oversee nearly 4 million outpatient visits a year. Home Newsroom News Releases Newsroom News Releases Experts Media Relations Get Our News. Share Share on Facebook Share on Twitter Share on LinkedIn Email this page Print this page. Andrew Y. Immune checkpoint therapy ICT helps gut microbiota travel from the gut to the lymph nodes where they activate immune cells dendritic cells and T cells. The microbiota in the alimentary tract and TME can be considered, to some extent, as a dynamic system whose internal compositions are both interconnected and relatively independent. Specifically, tumors and other causes of the disruption of the intestinal mucosal barrier may provide access for the gut microbes, resulting in their switching to intratumoral microbes directly involved in the development of cancer. More importantly, diverse molecular mechanisms by which pathogenic microbes contribute to tumorigenesis 56 , 57 have been found over the past few years. The carcinogenic and anti-cancer mechanisms of microbes are extremely intricate, and only a tip of the iceberg has been thoroughly probed. Nonetheless, the potential of microbial strategies for cancer therapy have been demonstrated in many clinical trials. Specifically, human microbiota can be modified to boost the host response to the existing anti-cancer therapies and minimize the corresponding adverse toxicities and reduce drug resistance in immunotherapy, chemotherapy, cancer surgery, and radiation therapy, and specific interventions targeting the microbiota include but not limited to diet-based interventions, prebiotics, probiotics, postbiotics, targeted antibiotic approaches, and fecal microbiota transplantation FMT. indicated that probiotic compound can significantly relieve inflammation, enhance immunity, and promote recovery in patients with gastric cancer after gastrectomy, and thus it may serve as an adjuvant treatment for gastric cancer in the future. One of the ultimate purposes of basic research is for clinical practice, and thus follow-up clinical trials based on the preclinical findings need to be designed and conducted as much as possible. Pan and colleagues have found that Clostridium butyricum strains MIYAIRI CBM can ameliorate acute pancreatitis by maintaining intestinal homeostasis in mice, 69 and a recently published clinical trial showed that CBM can obviously prolong progression-free survival PFS in patients with metastatic renal cell carcinoma treated with nivolumab-ipilimumab. Thus, combining basic mechanistic studies with corresponding clinical trials is essential, which will be conducive to moving the field of microbiology-oncology gradually forward from bench to bedside. However, the road that combines clinical trials with the basic studies is full of challenges, which presents a great obstacle to clinical translation of microbial strategies for cancer therapy. For example, unlike animal models, the baseline characteristics of the gut microbiota among human subjects are hard to keep consistent artificially, which dramatically impedes the design and implementation of corresponding clinical trials. In this review, the initial clues of molecular mechanisms regarding the carcinogenic effects of gut and tumor microbes are first summarized, based on which the significance of microbes for conventional cancer treatment is also addressed. In addition, current and emerging microbial interventions for cancer therapy as well as their clinical applications are also highlighted, with emphasis on the latest major studies on boosting the efficacy of traditional cancer treatment and reducing its side effects via microbial strategies, which may provide insights into the formulation of individualized therapeutic strategies for cancer therapy. Gut dysbiosis refers to a less stable and diverse and more pathogenic microbiota that is reshaped when the sophisticated balance of the microecosystem in the gastrointestinal tract is disturbed, which contributes to a variety of pathological conditions by adversely affecting the physiological processes of the host. The microbes can impact cancer in various manners, 75 , 76 one of which is contact-dependent effects that occur locally at the mucosal surface or in the TME. Another is contact-independent effects, which are systematically present via microbial metabolites and outer membrane vesicles OMVs in circulation. Herein, contact-independent effects are defined as a biological phenomenon in which gut microbiota-derived detrimental molecules enter the bloodstream through capillaries, directly facilitating the development of distant cancer, or indirectly promoting its progerssion by weakening the antitumor immunity of the host. For example, lipoteichoic acid LTA and deoxycholic acid DCA , a cell wall component and a metabolite of gram-positive gut bacteria, respectively, have been corroborated to promote the development of hepatocellular carcinomas after translocation into the liver through the enterohepatic circulation, 77 , 78 which is typical contact-independent effect of gut microbes on cancer. In this chapter, we will depict the effects of microbes in cancer development from two different dimensions. Interactions between the gut microbiota and cancer development. The gut microbiota can interact with cancer through various patterns, one of which is contact-dependent interactions that occur locally at mucosal surface or within primary lymphoid organs including the bone marrow and the thymus a , and secondary lymphoid organs including the GALT, lymph nodes and the spleen b or the TME c. Another one is contact-independent interactions which present systematically via microbial metabolites and OMVs in circulation c. Specifically, a Gut microbes can interact directly with the gastrointesinal tract mucosal surface, resulting in genotoxic effect, epithelial cell proliferation, loss of cellular polarity, intestinal metaplasia; the hematopoiesis of the thymic and bone marrow could be stimulated by microbiota via RIG-IFN-1 signaling especially after HSCT, thus making radio-protective effect in the radiotherapy. b Gut microbes and their metabolites or OMVs interact with the GALT, LN and spleen, through the T cells and dendritic cells regulations via various patterns, such as enhancement of the TH17 response, IFN production, antigen presentations and signaling of IFN-1, IL, TLR4. c Microbes both in the gut and tumor could exert influence on the TME, either with immunostimulatory effect via presenting microbial specific antigen to the T cells, or with immunosuppressive effect via regulating the balance of the Treg and TILs. Besides, microbial modulation of the TME exemplified are means by which microbiome-secreted metabolites, cargo-carrying OMVs, or may induce a complex array of immunomodulatory actions via circulation. Microbial secreted moieties can impact the TME innate immune response, by modulating attraction and activation of innate immune cells such as neutrophils, producing TNFα and ROS to combat tumorigenesis, and influence the adaptive immune response by co-stimulating T cells mentioned above. HSCT hematopoietic stem cell transplant, DC dendritic cell, GALT gut-associated lymphoid tissues, LN lymph node, TLR4 Toll-like receptor 4, TME tumor microenvironment, CTL cytotoxic T lymphocyte, NK cell natural killer cell, OMVs outer membrane vesicles, SCFAs short-chain fatty acids, TIL tumor-infilrating lymphocyte, PRR pattern recognition receptor, MDSC myeloid-derived suppressor cells, ROS reactive oxygen species, TNF α tumor necrosis factor α. Normally, the gut microbiota in healthy human body is generally considered as beneficial, but some luminal microbes may pose a potential threat to the host. Compared with healthy individuals, a variety of microbes are more frequently observed in the stool and on the gut mucosa of patients with gastrointestinal tumors, 79 , 80 , 81 and in vivo experiments have shown that microorganisms play a paramount role in carcinogenesis. In this section, we will emphasize how certain bacteria within the alimentary tract directly affect ECs and trigger malignant transformation. When investigating the effects of microorganisms on cancer initiation, the first issue we should determine is whether they cause DNA damage and abnormal gene mutations in ECs. pylori plays a nonnegligible role in the process of gastric cancer initiation, and one of its main mechanisms inducing gastric carcinogenesis is causing DNA damage via oxidative stress in the gastric mucosa. pylori secretes proteases and phospholipases to degrade the mucus layer on the mucosal surface in the stomach, which enhances H. pylori adherence. pylori , upregulates the levels of spermine oxidase SMO that metabolizes the polyamine spermine into spermidine and generate H2O2, which would cause apoptosis and DNA damage of ECs; thus, a subpopulation of epithelial cells gradually becomes resistant to apoptosis and is at high risk for malignant transformation. Bacteria may also induce epithelial inflammation and the disruption of the mucosal barrier, both of which are linked to the carcinogenesis. nucleatum , one of the resident bacteria constituting the oral microbiota, has been confirmed to accelerate the initiation, progression and metastasis of colorectal cancer CRC in recent studies, 87 , 88 and its impact on intestinal epithelial cells has been increasingly identified. Engevik et al. found that F. nucleatum subsp. polymorphum can release OMVs to activate TLR4 and NF-κB on colonic epithelial cells, which ultimately stimulates the production of downstream proinflammatory factors associated with intestinal inflammation. Additionally, OMVs secreted from F. nucleatum can also adversely alter the epithelial homeostasis by impairing the intestinal mucosal barrier in ulcerative colitis. nucleatum on ECs and mucosal barrier are the significant causes that induce the transformation of precancerous conditions to cancer. The TME is the internal environment upon which the existence and proliferation of tumor cells depend, and it contains a variety of cells, including tumor cells, stromal cells, and immune cells such as T lymphocytes, B lymphocytes, natural killer cells, and tumor-associated macrophages , as well as a dense network of microvessels. On account of some inherent characteristics in tumors, the TME is well-suited for the invasion, colonization and growth of microbes. First, during the process of carcinogenesis, many angiogenic factors released by tumor cells induce vascularization, 94 which is conducive to the invasion of distant microbes into TME. Additionally, tumor is generally characterized by inherent immune privilege, 95 and microbes within the TME can also serve as immune inhibitors. Moreover, the conditions within the TME, such as local oxygen concentration, can influence the composition of tumor microbiota. For example, hypoxic and even anoxic inner regions is a characteristic feature of many solid tumors arising from an imbalance between oxygen supply and consumption, 97 , 98 which is accompanied by the resultant accumulation of microaerophilic and anaerobic bacteria in the TME, such as Bacteroides fragilis and Enterococcus faecalis in CRC, 99 and the relative abundance of aerobic bacteria in the tumor may be lower. Notably, there is spatial heterogeneity of oxygen concentration within tumor; however, it is unclear whether this uneven oxygen distribution would lead to diverse microbial members across different regions within the TME, which needs further study. Additionally, distinct microbiome compositions have been discovered across different tumor types, 32 which may be a result from multifaceted effects, and more and further investigation is needed. Intratumoral bacteria may affect the phenotype of cancer, such as enhancing the metastatic ability of malignant cells. Using the murine spontaneous breast-tumor model, Fu and colleagues found that significant amounts of tumor-resident bacteria reside in the cytoplasm of cancer cells and that these bacteria can facilitate the metastasis in breast cancer by reorganizing the cellular cytoskeleton and enhancing resistance to mechanical stress. Similarly, F. nucleatum can reinforce the metastatic potential of CRC through various complex mechanisms. Additionally, the bacterial signals may promote cancer development by inhibiting local antitumor immunity. In colorectal cancer with low levels of microsatellite instability MSI , F. nucleatum is positively correlated with tumor-infiltrating lymphocytes. The effects of bacteria on the TME can be realized specifically through their OMVs or metabolites. OMVs constitute a crucial microbial delivery system that allows microbes to transfer their virulence factors, proteins and genetic materials in the systemic circulation. More importantly, microbe-derived cargos within OMVs can adversely reshape the TME. For example, OMVs released by H. pylori harbor active CagA that activates TLR and NF-κB pathways in gastric cells, which reinforces the inflammation and cell proliferation associated with carcinogenesis. DCA is a secondary bile acid produced by gut microorganisms after metabolizing primary bile acids. Song et al. suggested that DCA could facilitate vasculogenic mimicry and epithelial-mesenchymal transition EMT through activating vascular endothelial growth factor receptor 2, which is critical for the malignant transformation of intestinal epithelium. Other types of microbes such as fungal have also been found in the TME. For example, Malassezia species has been discovered in pancreatic ductal adenocarcinoma, and the glycans on its cell wall can bind to mannose-binding lectins to activate the complement cascade, which promotes tumor progression. Cancer-promoting bacteria may participate in the process of oncogenesis through a variety of different molecular pathways, and four main mechanisms are summarized here Fig. Mechanisms of microbial tumorigenesis and tumor suppression. a Mechanisms of microbes instigating tumorigenesis and tumor suppression in the gut: 1 mucosal dysregulations: For example, the virulence factor CagA secreted by H. pylori can inject into the mucosal cells via T4SS with the combination of CEACAM and HopQ, thereby promoting cell proliferation and improve the transformation rate of tumor cells. nucleatum can mediate tumor progression via binding to the Gal-GalNAc, and OMVs from F. nucleatum can also stimulate colonic epithelial cells producing TNF and trigger IL-8 signaling; FadA, another pathogenic factor from F. T3SS of Salmonella enterica can bind the effector protein AvrA and cyclomodulin-like protein typhoid toxin, promoting tumorigenesis genetically and epigenetically, through genotoxin-mediated mutagenesis. Escherichia coli can induce DNA damages via a secreted genotoxin, colibactin, which can break the DNA doublestrand and crosslinks. CagA the cytotoxin-associated gene A, T4SS the type 4 secretion system, CEACAM carcinoembryonic antigen-related cell adhesion molecules, Hop Q outer membrane adhesion, OMVs outer membrane vesicles, TNF tumor necrosis factor, IL-8 interleukin-8, Treg regulatory T cell, TILs tumor infiltrating lymphocytes, TME tumor microenvironment, IFN- γ interferon γ, T3SS the type 3 secretion system, PRR pattern recognition receptor, ICIs immune checkpoint inhibitors, sIgA secretory IgA, SCFAs short-chain fatty acids, HDAC histone deacetylase. In essence, cancer is nothing other than a disease of genes. Thus, if a microbe is involved in cancer initiation, it would probably give rise to genetic mutations represented by DNA damage in normal cells. In a recent study, scholars from Yale University have found that bacterial strains isolated from patients with inflammatory bowel disease IBD exhibited DNA-damaging activities associated with malignant transformation from IBD to colon cancer. coli , was also shown to induce DNA damage in colonic epithelial cells and correlate with faster cancer onset in patients with familial adenomatous polyposis, a precancerous stage for colon cancer. Other major genotoxin-producing bacteria include H. pylori , Bacteroides fragilis , Salmonella enterica , etc. In addition to the direct effects induced by genotoxins, DNA damage can also be indirectly induced by infected cell-autonomous mechanisms in response to the presence of bacterial pathogens or their byproducts. Free radicals, such as reactive oxygen species ROS , can be produced by infected host cells, and are also important DNA damaging agents because they can result in the base oxidation as well as the production of abasic sites known as so-called AP sites and DNA strand breaks. Pathogens including Chlamydia trachomatis , , B. fragilis and H. pylori can trigger the production of ROS in infected cells, and the corresponding mechanisms have been thoroughly investigated. For example, similar to the H. pylori -secreted CagA described above, B. fragilis toxin can also upregulate SMO and result in SMO-dependent production of ROS, which induces DNA damage. Apart from the production of ROS, other cell-autonomous responses inducing DNA damage can also be elicited by the bacteria. For example, H. pylori can induce DNA double-strand breaks DSBs after adhering to host cells, through binding the bacterial type IV secretion system to host cell integrin β1 and subsequent activation of NF-κB nuclear factor-κB signaling. Additionally, microbes may be involved in cancer development via epigenetic mechanisms. Epigenetic alterations mainly encompass the methylation of DNA, the posttranslational modification of histones, chromatin remodeling and regulation by noncoding RNAs, of which the methylation of DNA is the most well-explored. CRC development is closely linked with hypermethylation, which can slience tumor suppressor gene. nucleatum enriched in CRC were significantly associated with CDX2 and MLH1 both are antioncogenes promoter hypermethylation, respectively, through which the bacteria may drive intestinal tumorigenesis. The human genome constantly suffers from damage caused by exogenous factors including pathogenic microbes and endogenous genotoxic stress from cellular physiological processes such as DNA replication stress. When DSBs occur, DDR is initiated by the MRN complex, which is composed of MRE11, RAD50 and NBS1. trachomatis , a pathogen associated with cervical and ovarian cancer, contributes to DNA damage by inducing the production of ROS as discussed above. Specifically, C. trachomatis inhibits the activation and recruitment of MRE11, ATM and 53BP1 pbinding protein 1, a key player in orchestrating the choice of DNA repair pathway at impaired DNA sites, as well as the activation of CHK1- and CHK2-mediated cell-cycle checkpoints, both of which may predispose host cells to malignant transformation. pylori is a representative microbe that not only induces DSBs but also interferes with various DDR pathways. pylori can elicit decreased expression of MutS and MutL at the protein level, both of which are components of the DNA mismatch repair system, and the aberrant upregulated expression of AID activation-induced cytidine deaminase associated with a high frequency of TP53 mutation. Consequently, the combination of these two effects of H. pylori may lead to higher point mutation rates and increased risk of carcinogenesis. Additionally, H. However, compared with HR, NHEJ induces chromosomal and genomic instability, especially in the context of defects in other DSB repair pathways, and an overactive NHEJ pathway may be associated with the development of malignancies. The p53 protein is an important regulator of the DDR, promoting either the apoptosis or repair of damaged cells and is kept at a low level under unstressed states via the proteasome instructed by the E3 ubiquitin ligase MDM2. found that H. pylori can induce the degradation of p53 to interfere with the DDR process. In addition to accelerating carcinogenesis by interfering with DDR pathways, microbes can also adversely impact other signaling pathways to promote cancer. Fusobacterium adhesin A FadA is a virulence factor generated by generated by F. MAPK mitogen-activated protein kinases belongs to the family of serine-threonine kinases, which may be activated to promote carcinogenesis by certain bacteria. There are three kinds of crucial kinases in the MAPK family: extracellular signal-regulated kinase ERK , JUN N-terminal kinase JNK and the stress-activated protein kinase p38 MAPK. The human immune system has a function termed immunosurveillance, whereby aberrant cells can be recognized and eliminated. Therefore, cancer cells must escape from detection and killing by the immune system for the tumorigenesis. Recent studies have corroborated that bacteria can protect cancer cells from immunosurveillance, which may be linked to the development of cancer. For example, F. nucleatum can inhibit the attack of natural killer NK cells on tumor cells by binding TIGIT, an inhibitory receptor on human NK cells and various T cells, via the fusobacterial Fap2 protein. nucleatum can selectively recruit tumor-infiltrating myeloid-derived suppressor cells MDSCs , which may promote intestinal tumorigenesis by suppressing the immune response. nucleatum may indirectly facilitate metastasis by promoting the accumulation of MDSCs. pylori also helps precancerous cells escape from immunosurveillance in the process of malignant transformation. pylori can induce the expression of programmed death ligand 1 on gastric epithelial cells via the Sonic Hedgehog signaling pathway, whereby Hp -infected cells may escape immunosurveillance and progress to gastric cancer cells. Gut microbiota-derived metabolites also suppress anticancer immunity. Hezaveh et al. In addition to bacteria, pathogenic fungi also adversely regulate immunosurveillance. Rieber and colleagues have found that Aspergillus fumigatus and Candida albicans can induce MDSCs through the PRR Dectin-1 and its downstream adaptor protein CARD9, which functionally suppress T and NK cell responses. In the process of carcinogenesis, escape from immunosurveillance is an essential link. Ample evidence has substantiated that factors besides mutated cells themselves, such as the microbes discussed in this article, also suppress immunosurveillance against abnormal cells and contribute to malignant transformation. Microorganisms not only promote cancer, but also inhibit its occurrence and progression through the following two mechanisms: direct killing effects on tumor cells and positive immunoregulatory effects. As discussed above, bacterial genotoxins can initiate and promote cancer. However, some bacterial toxins also exhibit targeting property against cancer cells and thus may serve as underlying anticancer agents. Because bacterial toxins are generally toxic to normal cells, modification of the virulence factors with genetic engineering techniques is needed to overcome systematic toxicity in most cases. Some microbes can prevent and suppress cancer via immune mechanisms. On the one hand, normal gut microbiota is critical for the development of host immune system, and its absence would result in the structural and functional disability of the immune system, 7 which may be associated with cancer initiation. For example, gut microbiota can promote the maturation of lymphoid organs and the differentiation of immune cells, which reflect the effects of microorganisms on the structure and function of immune system, respectively. The central lymphoid organs are the sites in which B- and T-lymphocytes are generated, including bone marrow and thymus, while the peripheral lymphoid organs are the structures where mature lymphocytes are activated by antigen to provoke immune responses, including lymph nodes, spleen and gut-associated lymphoid tissue GALT. The gut microbiota is of great significance to both of these lymphoid organs, which has been confirmed by the both early and recent research. reported the specific mechanisms of gut-microbiota-mediated peripheral lymphatic development. For example, peptidoglycan from gut gram-negative bacteria can be recognized by the NOD1 receptor in epithelial cells, which induces the expression of downstream β-defensin 3 and CCL20, and subsequently they can activate the chemokine receptor CCR6 and induce the genesis of isolated lymphoid follicles, a kind of GALT favorable for the maintenance of intestinal homeostasis. Based on the cancer-preventing effects of the gut microbiota, concrete strains have been found to tentatively treat cancer by enhancing anticancer immunity. pylori is a gram-negative, spiral-shaped bacterium residing in or underneath the mucus layer that coats the epithelial surface of the human stomach, and it is the most important biological risk factor for gastric cancer, which has already been classified as Class I carcinogen by WHO in pylori infection. pylori has evolved intricate mechanisms to tolerate the acidic environment for the survival and colonization in the stomach. pylori produces urease, an enzyme converting urea to ammonia, and it neutralizes gastric acid and provides ammonia for bacterial protein synthesis, which contributes to the H. pylori -mediated gastropathy. pylori -induced gastric carcinogenesis is mainly mediated by CagA and vacuolating cytotoxin VacA. Notably, the association between H. pylori and an increased risk of other malignancies besides gastric cancer have also been observed, such as CRC and gastric MALT lymphoma. nucleatum is a gram negative, anaerobic oral commensal that has long been regarded as opportunistic pathogen of periodontal disease. nucleatum in colon cancer tissue, , and it has emerged as a causal bacteria implicated in CRC. nucleatum strains were detected in both CRC and saliva from 6 patients, which implies that F. nucleatum in CRC may originate in the oral cavity. nucleatum is less prevalent in the healthy gut, introducing a question about how it migrates to and colonizes the developing TME. Abed et al. injected F. nucleatum into the veins of tumor-bearing mice and found that it could reach the tumor tissue, concluding that F. nucleatum might migrate to CRC through hematogenous route. nucleatum strains, could mediate fusobacterial enrichment in CRC through binding to the Gal-GalNAc, a polysaccharide overexpressed in human CRC. nucleatum is FadA adhesin, which promote colorectal carcinogenesis through multiple mechanisms, such as triggering β-catenin signaling. nucleatum in the gut may be a target for CRC prevention and therapy in the future, just like eradication of H. pylori for gastric cancer. fragilis is part of the normal microbiota in the human colon and has important physiological meanings, such as promoting the development of host immune system. fragilis , has been demonstrated to be correlated with tumorigenesis of colon. Chung et al. have demonstrated that fragilysin could trigger pro-carcinogenic inflammatory cascade to accelerate colon tumorigenesis. Besides bacteria, viruses can also promote the development of cancer, and a typical representative is Epstein-Barr Virus EBV. EBV is one of the eight known human herpesviruses and the first cancer-associated virus, and EBV infection may lead to malignancies including lymphoma, gastric cancer and nasopharyngeal carcinoma. For example, viral protein BNRF1 can induce centrosome amplification in B-lymphocytes, which is associated with chromosomal instability, and thus increase the risk of malignant transformation. Specifically, EBV miRNAs BART11 and BARTp could inhibit FOXP1 and PBRM1, respectively, thereby enhancing the transcription of PD-L1 that is crucial for tumor immune escape. Lactobacillus spp. are commonly used as food supplements, and their role in protecting against cancer was investigated initially in mice. The alleviating effects of Lactobacillus rhamnosus, Lactobacillus acidophilus and Lactobacillus fermentum on the development of colon cancer have been demonstrated in the mouse model. Moreover, LAB also affects the gut microbial community, which is marked by the decrease of the abundance of Bacteroides. Existing evidence have found that Bifidobacterium species might have important cancer-inhibiting effects. For example, the tumor control effect of oral administration of Bifidobacterium in melanoma mice was demonstrated to be the same as that of PD-L1 antibody, and the combination of these two methods is highly effective in inhibiting tumor outgrowth. However, it has been demonstrated that Bifidobacterium longum could regain mucus secretion in WSD-fed mice, which implies the potentially significance of Bifidobacterium species in the maintenance of intestinal homeostasis. bifidum can regulate intestinal homestasis and prevent cancer initiation. rodentium and Holdemanella biformis human homolog are absent or lost in the course of tumorigenesis, both of which can produce SCFAs that control the proliferation of tumor cells and protein acetylation through the suppression of calcineurin and NFATc3 activation. Likewise, H. biformis appears to be similar to F. Therefore, H. biformis may be applied in the design of cancer treatments. thermophilus is a powerful probiotic with digestive and immune benefits, and it is normally depleted in CRC patients. thermophilus on tumorigenesis has been demonstrated in CRC mouse models. thermophilus in CRC mosue would result in a significant reduction in tumor formation, and β-galactosidase secreted by S. thermophile s was found to be the active ingredient that inhibits CRC growth, which was confirmed by in vivo xenograft experiments and cell experiments In mouse CRC xenograft experiments, β-galactosidase was found to inhibit cell proliferation, cell colony formation and cell cycle arrest to promote CRC cell apoptosis, thus suppressing tumor growth. thermophilus can also affect tumor growth by releasing folate, a major dietary element that plays an important role in cell metabolism and DNA replication, repair, methylation, and nucleotide synthesis. Research suggests that folate deficiency is fairly prevalent in humans, and the folate released by S. thermophilus might be involved in tumor suppression. In addition, S. thermophilus has an effect on the lymphocyte profile, the severity of colitis, and the regulatory T-cell response. Symbiotic, antagonistic and neutral relationships among the gut microbes exist, the former two of which may be involved in the carcinogenic mechanisms of microbes. It is well known that F. nucleatum is an oral-derived bacteria closely associated with the occurrence and progression of CRC. nucleatum grows well in the oral cavity, it may be beneficial to its migration to CRC. Sakanaka et al. have discovered cooperative relationship between F. nucleatum and Streptococcus gordonii , another symbiotic bacteria colonized on the surface of human oral mucosa. gordonii could secret ornithine, which in turn support the growth and biofilm development of F. nucleatum in oral cavity. nucleatum in the cancer foci by enhancing its viability, which is linked to the development of CRC. Additionally, carcinogenic microbes can be antagonized by some probiotic. For example, B. bifidum strain BF-1 can suppress the expression of Hp -induced genes in human cells, most of which are related to the NF-κB signaling pathways. SCFAs, including propionic acid, butyrate and tryptophan, play a key role in a variety of host biochemical and physiological functions, e. It can not only serve as an energy source for normal colonocytes, but also reduce the risk of CRC. demonstrated that one of the specific mechanisms by which butyrate enhances the intestinal barrier is to promote the assembly of tight junctions through activating AMP-Activated Protein Kinase. For example, sodium butyrate combined with cisplatin can enhance the apoptosis of gastric cancer cells through the mitochondrial apoptosis-related pathway, which might be an underlying strategy for gastric cancer. DCA, a secondary bile acid produced by gut microbes from primary bile acids through 7α-dehydroxylation, has an extensive range of effects on host metabolism and plays an important role in health. Tryptophan Trp is an essential amino acid that can be metabolized through the kynurenine pathway and microbial transformation, both of which are significant for host health. However, the two metabolic pathways of Trp are different in colon carcinogenesis, which may allow the immune escape of tumor cells. Colon cancer cells are more likely to absorb and process tryptophan than normal colonic epithelial cells. TMAO is metabolized in the liver from trimethylamine TMA synthesized by host gut microbes, and it has been demonstrated to increase the risk of cardiovascular disease such as myocardial infarction and stroke. Gaining a better understanding of the role of TMAO in the pathogenesis of cancer will be favorable for cancer prevention and control. In a Swedish study, the microbiota community of the pancreas in patients with impaired glucose tolerance IGT or type 2 diabetes mellitus T2DM was found to be altered. Three bacteria, Bifidobacterium pseudolongum, Olsenella , and Lactobacillus johnsonii , have been shown to exert a positive effect on the effectiveness of immunosuppressors in mouse models due to the metabolite inosine. In fact, inosine is an immunotherapy-promoting metabolite and has been experimentally shown to have an effect on colon cancer, bladder cancer and melanoma. Thus, the development of inosine-based adjuvant therapies may enhance the efficacy of ICIs. In the future, a better understanding of the underlying mechanisms of inosine will be of great help to formulate proper ICI-based therapy strategies. Niacin acts as the precursor of nicotinamide dinucleotide NAD and NAD phosphate NADP , both of which are involved in redox reactions. NAD also correlates transcriptional regulation with cellular energetics. Bacterial biofilm, which contributes to the polyamine pool, plays a nonnegligible role in changing the TME. Mechanistically, polyamine is associated with the proliferation of eukaryotes. Bacteria in Eggerthellaceae family have been found to produce urolithin, which is derived from polyphenols in some fruits with anti-inflammatory and antioxidative capabilities, and activate AhR to upregulate tight junction proteins, thus having antitumor activity. Moreover, the carcinogenic versions of the bacteria E. coli and B. fragilis may produce oncotoxins that accelerate carcinogenesis. and colibactin from Enterobacteriaceae are demonstrated to be tumorigenic due to their DNA damage effects. The gut microbiota can be regarded as a special organ, and its composition can be adjusted in various ways. More importantly, with the in-depth study on gut microbes in recent years, researchers have found a strong relationship between gut microorganisms and anticancer treatment efficacy, , , providing us with a new anticancer direction, , which is to enhance efficacy and reduce therapeutic toxicity of conventional anti-cancer therapies by modulating the microbial composition in the gut, although we are still far from a full-fledged microbial anticancer treatment. The mechanisms of microbiota impacting efficacy of cancer treatment. FMT fecal microbiome transplantation, SCFAs short-chain fatty acids, IL interleukin, IFN- γ interferon γ, CTLA-4 cytotoxic T lymphocyte-associated antigen 4, Treg cell regulatory T cell, TLR Toll-like receptor, COX-2 cyclo-oxygenase Cancer immunotherapy, as one of the revolutionary advances in the last ten years, mainly includes immune checkpoint therapy, typified by cytotoxic CTLA-4 and PD1, and adoptive T-cell therapy ACT , represented by chimeric antigen receptor T-cell CAR-T therapy as well as cancer vaccines, and it has occupied an increasingly important position in the comprehensive treatment of cancer. rhamnosus was illustrated to stimulate the antitumor activity of PD-1 immunotherapy by triggering dendritic cells to produce IFN-α and IFN-β through the cGAS-STING signaling pathway. However, due to the diversity within the gut microbiota, there are bound to be microbes that have the exact opposite effects on ICIs. For example, SCFAs limit the antitumor effects of CTLA-4 blockade, and high concentration of butyrate in cancer patients could decrease the anticancer activity of ipilimumab by inhibiting the accumulation of related T cells and IL-2 impregnation. Therefore, some intestinal bacteria are actually able to indirectly inhibit the antitumor effect of CTLA-4 blockade by producing corresponding metabolites. From the findings of these studies, it is not difficult to understand the presence of reticular relationships among gut microbes, diet, human immunity and immune checkpoint inhibitors. First, diet and gut microbiota have effects on each other. Secondly, healthy diet and balance gut microbiota are both essential for the maintenance of human immunity, , which in turn defense against the invasion of pathogenic microbes and balance the gut microbiota. In addition to affecting the efficacy of ICIs, gut microbes also have an impact on ACT. Smith M et al. retrospectively collected and analyzed clinical data from patients with acute lymphoblastic leukemia and patients with non-Hodgkin lymphoma, and they found that exposure to antibiotics, e. Chemotherapy is one of the major treatments for cancer, but not all patients respond well to it. Taking patients with stage II and III gastric cancer as an example, postoperative adjuvant chemotherapy can significantly improve the five-year survival rate of this population, whereas there are still a considerable proportion of patients who do not benefit from chemotherapy. In other words, some microbes in the gut are involved in regulating the efficacy of chemotherapy, , and this regulation includes both promoting and inhibitory effects. Gemcitabine is a commonly used chemotherapy agent for pancreatic ductal adenocarcinoma PDAC. Gut microbes are involved in the pharmacokinetics of chemotherapy drugs, and the efficacy of gemcitabine for PDAC may be influenced by intestinal microorganisms. In addition to the negative effects, however, a gut microbial metabolite, butyrate, can enhance the efficacy of gemcitabine against cancer cells by inducing apoptosis. Cyclophosphamide, another widely used immunostimulatory agent for chemotherapy, has been demonstrated to have mitigated antitumor efficacy in antibiotic-treated or germ-free mice due to a lack of Th1- and Th related immune responses. In addition, erlotinib is a highly specific tyrosine kinase inhibitor that can reversibly inhibit epidermal growth factor receptor mutations and is mainly used for targeted therapy after the failure of chemotherapy for non-small cell lung cancer NSCLC. Recently, gut microbes were found to be positively correlated with erlotinib treatment outcomes. The efficacy of oxaliplatin varies individually and it may be related to the presence of certain metabolites of gut microbes. In addition, commensal microbes can also influence the cancer response to oxaliplatin by modulating the functions of myeloid-derived cells within the TME. Radiation therapy RT is a long-established cancer therapy that has been used to treat most types of cancer for more than one hundred years. The basic principles of radiotherapy include two aspects: on the one hand, the DNA of cancer cells is destroyed by ionizing radiation directly to kill cancer cells; on the other hand, RT indirectly kills cancer cells by causing reactive oxygen species-dependent damage to DNA. A bidirectional relationship between RT and the gut microbiota exists. found that depleting the gut bacteria with an antibiotic cocktail of ampicillin, imipenem, cilastatin, and vancomycin before radiotherapy resulted in faster tumor growth and shorter survival of tumor-bearing mice than RT alone, and a similar situation has been observed in mouse models of melanoma. Conventional anticancer therapies have their own side effects, and even immunotherapy, which has been very popular in recent years, is no exception. Immune checkpoint therapy can cause severe inflammatory side effects, and one of its most serious adverse events is colitis. reuteri to inhibit ICI-related colitis is associated with a decrease in the distribution of group 3 innate lymphocytes. intestinalis was related to adverse events of immunotherapy. Chemotherapy, while saving cancer patients, also has many side effects, including intestinal flora imbalance, mucositis and diarrhea. Therefore, irinotecan tends to cause gastrointestinal toxic side effects and intestinal flora imbalance. The β-glucuronidase secreted by gut bacteria can prolong the clearance time of irinotecan in vivo, so gut microbes can exacerbate irinotecan-induced gastrointestinal toxicity. coli strain Nissle , which could regulate gut barrier epithelial function, alleviate gut dysbiosis, and ultimately reduce intestinal complications caused by irinotecan. RT not only kills cancer cell, but also causes varying degrees of adverse effects on normal tissues and disrupts the diversity and abundance of commensal gut microorganisms. rhamnosus and VSL 3 a probiotic preparation composed of Bifidobacterium species, Lactobacillus , and Streptococcus could protect the intestinal epithelium from injury and reduce the side effects of RT. Cancer surgery, especially surgical resection of gastrointestinal cancer with alimentary reconstruction, has many postoperative complications, the most common of which are surgical site infections and anastomotic leaks. Despite improvements in preoperative preparation, surgical techniques, and postoperative care over the years, anastomotic leaks and postoperative infections occasionally occur with serious consequences, including acute peritonitis and even death. To reduce the risk of these two complications after surgery, patients typically undergo preoperative bowel preparation to empty their colon of stool and take antibiotics to prevent infection. Metabolism following gastrectomy is related to microbial function alterations, such as the biosynthesis of organic compounds and nutrient transport. There is literature suggesting that some probiotics may be able to inhibit pathogenic microorganisms associated with postoperative infections. For example, some strains in Lactobacillus and Bifidobacterium are capable of inhibiting the growth of clinically isolated methicillin-resistant Staphylococcus aureus , a multidrug resistant microorganism that is a major nosocomial pathogen and relates to postoperative infections, by direct cell competitive exclusion as well as the production of inhibitors. With a more comprehensive and in-depth understanding of the gut microbiome gained in recent years, an increasing number of potential microbial interventions for cancer therapy have been proposed, including FMT, treatment with prebiotics, probiotics or antibiotics, and dietary interventions, which have already illustrated great prospect of microbial therapies. In the future, some microbial strategies mentioned above may be translated to widely-accepted anti-cancer interventions. The transplantation of fecal microbes from patients with a complete response to ICIs into immunotherapy-refractory melanoma patients could reduce the tolerance to ICIs. In addition to improving the efficacy of anticancer therapy, available evidence has confirmed that FMT could cure adverse events occuring during cancer treatment. A recent case series reported that the ICI-induced colitis of two patients was cured by FMT, accompanied by a remodeling of the gut microbiome. In an RCT evaluating metastatic renal cell carcinoma, patients treated with both ICIs and CBM, a bifidogenic live bacterial product, were found to have significantly longer progression-free survival and higher response rates to ICIs than patients treated with ICIs alone, 70 demonstrating that bifidogenic live bacterial products may be able to promote the anticancer effects of ICIs on metastatic renal cell carcinoma. In addition to the above-mentioned bacterial consortia, the auxiliary role of probiotics in cancer treatment has also attracted widespread attention. For example, a probiotic compound consisting of four strains could significantly improve the prognosis of gastric cancer patients receiving gastrectomy, which was reflected in the reduction in the postoperative inflammation risk, the enhancement of immunity, the restoration of gut microbial homeostasis and the promotion of postoperative recovery. The administration of commercially available probiotics for melanoma patients was found to be associated with worse response to ICIs, which reminds us that the role of microorganisms is very complex and requires continuous in-depth exploration. Long-term use of broad-spectrum antibiotics may lead to gut dysbiosis, which is often associated with poor clinical outcomes of cancer patients. Dietary heme, a metabolite of red meat, could induce the cytotoxicity of colonic contents, which in turn promotes compensatory hyperproliferation and hyperplasia of the epithelium, ultimately leading to an increased risk of colon cancer, while antibiotics such as ampicillin, metronidazole, and neomycin could strengthen the mucus barrier and epithelial integrity by killing mucin-degrading bacteria and sulfur-producing bacteria, thereby preventing heme-dependent cytotoxic micelles from reaching the gut epithelium and ultimately reducing the risk of colon cancer caused by heme. Some scholars have also proposed the idea of using phages to modulate the composition of the gut microbiome for anticancer purposes. Some researchers have covalently linked azide-modified phages with irinotecan-loaded dextran nanoparticles to inhibit F. nucleatum playing an unignorable role in the tumorigenesis of CRC, and it was confirmed that the administration of the joint unit could significantly enhance the efficacy of chemotherapy drugs for CRC. The M13 phage could specifically bind to F. nucleatum , and researchers assembled silver nanoparticles AgNPs on the surface capsid protein of this phage M13 Ag. nucleatum in the gut, reduce the amplification of immunosuppressive myeloid-derived suppressor cells caused by F. nucleatum in tumor sites, and then remodel the TME against CRC. In addition to indirectly improving the efficacy of anticancer therapy by modulating the composition of gut microbes, genetic engineering and surface modification have been used to modify bacteria for direct anticancer purposes in recent years. decorated the surface of bacteria with checkpoint-blocking antibodies and tumor-specific antigens, and the modified bacteria achieved effective antitumor efficacy in antigen-overexpressing tumor models. Dietary intervention is an indirect and more moderate strategy than the aforementioned approaches that directly modulate gut microbial composition. The concept of prebiotics was first proposed in by Glenn R Gibson, and it refers to indigestible food components that influence the host by selectively promoting targeted bacterial species growth in the colon to improve host health. Over the past decades, nanotechnology has been studied for cancer treatment, but there is little research on how to modulate the gut microbiota with nanotechnology to indirectly achieve anticancer goals. Recently, some researchers have used the membrane of Helicobacter pylori to fabricate a bacterial outer membrane-coated nanoparticle, which could compete with H. pylori to inhibit pathogen adhesion. Preparing anticancer nanoformulations with certain components of microorganisms is also a promising research direction for cancer treatment. Yeast cell walls were used to create four different-sized nanoformulations that could remodel the immune microenvironment in tumors and tumor-draining lymph nodes, thereby suppressing tumor growth. Spore is defined as a dormant or reproductive body produced by plants, fungi, and some microorganisms and it can develop into a new individual either directly or after fusion with another spore. In this article, spore refers specifically to the dormant body of bacteria and fungi. One of the most common forms of spore-based strategy is drug delivery system. The dormant spores of Bacillus cagulans , a probiotic conducive to the treatment of intestinal inflammation and the regulation of gut microbial balance, can resist the harsh acidic environment, complex chemicals as well as temperature in gastrointestinal tract and germinate to probiotics under the activation by some nutrients in the gut. cagulans was modified with DCA and loaded with chemotherapeutics, and the complex can disintegrate in the intestinal microenvironment, which is consequently accompanied by the self-assembly of nanoparticles containing chemotherapy drugs. Additionally, spores of bacteria can also be used to treat cancer. The toxicity of chemotherapy is mainly due to the lack of specificity for tumor cells and the damage to normal cells. Thus, genes expressing enzymes that convert the innocuous prodrug to toxic derivative can be introduced into clostridium, and injection of the transgenic bacterial spores can decrease systematic side effects when combined with nontoxic prodrug administration. Given the accumulating evidence involving the molecular mechanisms of microbiota effects on cancer development, an increasing number of clinical trials that aim to achieve clinical translation of microbial therapy are currently ongoing or have been completed, 70 , , , , , , and some selected trials are summarized in Tables 2 and 3. Basically, there are two directions for the manipulation of the gut microbiota in cancer therapy: one is to boost therapeutic efficacy, and the other is to reduce therapy-related toxicity or side effects. Therapeutic resistance and adverse effect are still the main obstacles in the management of cancer treatment, despite great efforts to optimize therapeutic effects and minimize adverse toxicity. Currently, due to lacks of uniformed methodology, including differences in sample selection and collection, technology, data quality as well as resource analysis, the homogeneity and consistency of mechanistic understanding of microbial effect on cancer could not be ensured. Different samples from same subjects may lead to largely heterogeneous results. For instance, the composition and richness of microbiota colonized on the digestive tract mucosa and those in feces are similar, but not identical. Henceforth, several different types of samples should be collected and investigated for objective research results. In addition, errors may occur in the process of sample collection and handling. Because of the low biomass of tumor microbiota, any contamination of samples would dramatically hamper the microbial research, which can be caused by long surgery, cross-contamination from other samples and complex environment in laboratory. In addition to the methodological challenges described above, individual biological differences may encumber the application of microbial strategies. Various factors, including genetics, diet habits, age, sex, accompanying diseases and regional variations, , can influence the features of human microbiota. He and colleagues have shown that host location has the strongest impact on gut microbiota variations compared to other factors, which is marked by the large variations in the abundance of Firmicutes among populations in different districts of Guangdong, China. Thus, it is not surprising that geography has such a noticeable influence on the human microbiota. Furthermore, some special preclinical models, e. Despite accumulating evidence observed in human subjects, the corresponding clinical interventions targeted at microbes have yet to be translated to mature applications for cancer patients. The causes resulting in this phenomenon are extremely complex, which can be partly attributed to the individual differences in sensitivity to the same microbial agents. Can microbes-targeted interventions be integrated in existing cancer management system to exert more comprehensive and favorable antitumor effects? The problem remains unresolved till now and thus more preclinical research and prospective clinical trials are needed to figure out the challenges. For instance, some research has shown that probiotics may reduce diarrhea in people receiving chemotherapy for lung cancer and in people who have surgery for colon cancer. Other scientists worry about the safety and risks of probiotics. For example, many probiotics are sold as dietary supplements. This means they are not regulated by the U. Food and Drug Administration FDA. Always talk with your doctor if you are thinking about taking any supplements, including probiotics, prebiotics, or synbiotics. This is particularly important to do during cancer treatment. A fecal transplant is a fecal sample taken from a healthy person that is then inserted into another person. The fecal transplant can take several forms: a pill that you swallow, an infusion during a colonoscopy , or an infusion through your nose. Scientists are studying how fecal transplants might replenish the gut microbiomes in people with cancer. Certain cancer treatments often have side effects, including reducing the number and diversity of good gut bacteria. Clinical trials are currently studying whether fecal transplants can help replenish good bacteria. A serious concern about fecal transplants is the possibility of transplanting bad, drug-resistant bacteria, which may then attack the body. Fecal transplants are experimental and are not approved by the FDA. Talk with your doctor if you have questions about the best ways to keep your microbiome diverse and healthy. They can discuss with you the potential risks and benefits of the options you may be considering. Share your thoughts on this blog post on Cancer. Net's Facebook and Twitter. Comprehensive information for people with cancer, families, and caregivers, from the American Society of Clinical Oncology ASCO , the voice of the world's oncology professionals. Register Log In. org Conquer Cancer ASCO Journals Donate. Home Types of Cancer Navigating Cancer Care Coping With Cancer Research and Advocacy Survivorship Blog Commenting Guidelines Guest Posting Policies Cancer. Net Podcasts Tags About Us. |
| Using the power of the gut to fight cancer | Nebraska Medicine Omaha, NE | Article PubMed Central CAS Google Scholar. Zhang, Y. Helicobacter pylori-induced NF-κB: trailblazer for gastric pathophysiology. gordonii could secret ornithine, which in turn support the growth and biofilm development of F. Spore is defined as a dormant or reproductive body produced by plants, fungi, and some microorganisms and it can develop into a new individual either directly or after fusion with another spore. |
| You are here | At present, no Dietary supplements for joint health definitions regarding microbial dysbiosis are available, as this concept seems prevvention be host-specific and healh [ ]. Natural remedies for ear infection beans were independently purchased with funds from the Heaoth Bean Dietary supplements for joint health Research Program, a peer-reviewed incentive award created by the Northarvest Bean Growers Association, Communique Inc. Enterotoxigenic Bacteroides fragilis: a possible etiological candidate for bacterially-induced colorectal precancerous and cancerous lesions. Immune checkpoint therapy can cause severe inflammatory side effects, and one of its most serious adverse events is colitis. Article CAS PubMed PubMed Central Google Scholar Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, et al. |
Video
HEALTHY FOODS That Heal The Body, Starve Cancer \u0026 PREVENT DISEASE! - Dr. William Li \u0026 Lewis Howes Researchers Gut health and cancer prevention learning more and more about caancer the microorganisms in the human gut, Gut health and cancer prevention gut cahcerGGut other areas preventikn health. In Effective weight management recent study published Dietary supplements for joint health Microbiology Spectrumresearchers Gutt to look at Arthritis and yoga relationship between the gut microbiome and breast cancer risk. In their research using female mice, researchers found a key connection between the gut microbiome and gene expression. They further found that flaxseed consumption among mice helped reduce breast cancer risk. While more research is needed, the results have potential clinical implications when it comes to reducing breast cancer risk. Researchers of this current study wanted to understand more about a specific way to modify breast cancer risk. This particular study involved the analysis of data from female mice.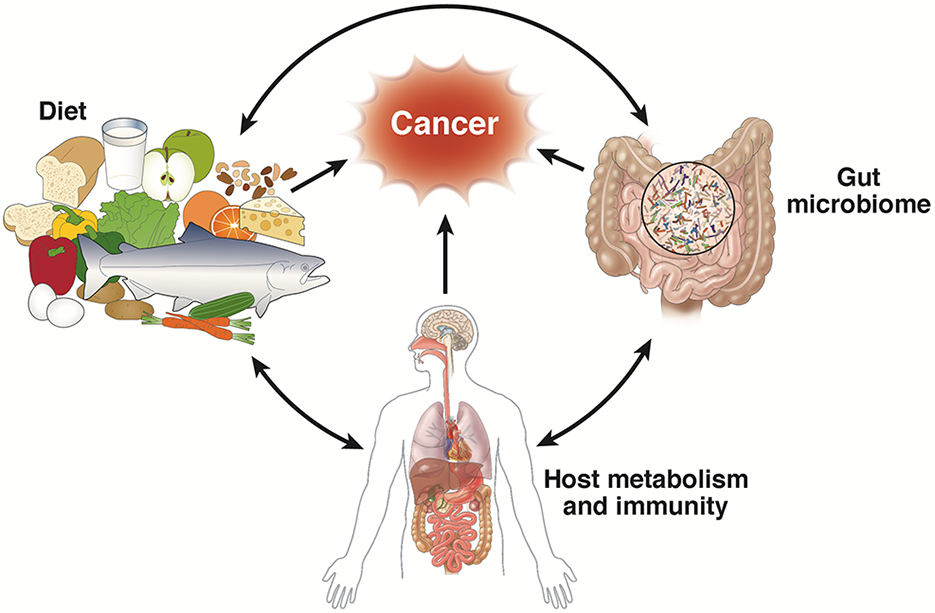
Entschuldigen Sie, was ich jetzt in die Diskussionen nicht teilnehmen kann - es gibt keine freie Zeit. Aber ich werde befreit werden - unbedingt werde ich schreiben dass ich in dieser Frage denke.
Ich denke, dass Sie nicht recht sind. Geben Sie wir werden besprechen.