Video
Top 10 Most HARMFUL Foods Thought As HEALTHYGlucose metabolism pathways disorders -
The absence of insulin signaling in diabetic patients leads to disruption of this mechanism, ultimately leading to diabetic cardiomyopathy. Dissociation of HK2 from VDAC leads to opening of mPTP and release of cytochrome C into the cytoplasm, causing apoptosis. Phosphoglycerol metathesis PGAM is a glycolytic catalase that catalyzes a reversible reaction between 3-phosphoglycerate 3-PGA and 2-phosphoglycerate 2-PGA.
During the neonatal period, the main source of energy required for cardiac metabolism gradually changes from glycolysis to fatty acid oxidation, during which PGAM expression gradually declines [ 89 ].
PGAM is a dimeric enzyme with two isoforms in mammals, the non-muscle isoform type B and the muscle-specific isoform type M [ 90 ]. Three isoforms can be composed: MM-PGAM, MB-PGAM, and BB-PGAM. BB-PGAM, named PGAM1, was originally isolated from the brain and is highly expressed primarily in the brain, kidney, and liver [ 90 ].
MM-PGAM, named PGAM2, is a muscle-specific form that is highly expressed mainly in skeletal muscle and heart. Its activity is regulated by several post-translational modifications. Among them, sumoylization is an important mechanism for regulating PGAM2 activity.
The two main SUMO receptor sites are K49 and K, and mutations in these key sites may be associated with glycogen storage disease X GSDX or other mutation-associated diseases [ 91 ].
MB-PGAM is predominantly expressed in the heart. The heart is the only tissue that contains all three forms of PGAM [ 92 , 93 ]. It plays an important role in the regulation of cell death and mitochondrial dynamics [ 94 ].
The major signaling pathways involved include the typical pathway RIP3-MLKL pathway as well as the atypical pathways RIP3-CaMKII-mPTP pathway, RIP3-PGAM5-Drp1-mitochondrial pathway, and RIP3-JNK-BNIP3 pathway [ 95 ].
Indeed, the RIP3-MLKL pathway is the most studied mode of necroptosis induction in necroptosis, however, other atypical pathways such as the RIP3-PGAM5-Drp-1 axis are also receiving increasing attention [ 96 ].
It has been reported that a protein complex containing RIP1 and RIP3 is formed after necroptosis induction, and one of the components of this complex is PGAM5.
PGAM5 has two spliceosomes, PGAM5L long form and PGAM5S short form , and both play roles in the necroptosis pathway [ 97 ]. When RIP3 translocates to mitochondria, PGAM5 expression is elevated, and PGAM5S recruits the mitochondrial fission factor Drp1.
It also promotes dephosphorylation of Ser on Drp-1, which in turn leads to mitochondrial fission, elevated ROS, and ultimately cell death.
Some researchers have claimed that while inhibition of RIP3 prevents plasma membrane rupture early during reperfusion, it does not appear to rescue cardiac dysfunction by affecting these types of necroptosis signaling but is more likely to be related to mitochondrial oxidative stress regulated by RIP3 via xanthin oxidase XO and manganese superoxide dismutase MnSOD [ 95 ].
Taken together, PGAM5 can serve as a novel inducer of necroptosis, providing new possibilities for the treatment of myocardial infarction. In addition, since RIP3 is the confluence of these signaling pathways, reducing the nuclear translocation of RIP3 is also a new idea [ 95 , 98 ].
Thus, this atypical necroptosis signaling appears to be more complex and requires further study. A growing body of research suggests that metabolic reprogramming is an early alteration in heart failure and correlates with the severity of HF [ 14 ]. PGAM2 has the second highest level of activity in the heart after skeletal muscle [ 91 ].
An earlier study observed an approximately fivefold increase in PGAM2 protein expression in a canine model of tachycardia-induced heart failure [ ]. Similarly, Li et al. detected elevated PGAM2 expression in the serum of HF patients. It also illustrates that PGAM2 is a new biomarker for assessing the severity of HF with an accuracy comparable to BNP [ ].
A recent report states that sustained overexpression of PGAM2 can alter the levels of metabolites in glycolysis and the TCA cycle and may alter fatty acid and mitochondrial metabolism, which may disrupt mitochondrial function and increase cardiac stress sensitivity [ ].
This predisposes the heart to severe myocardial fibrosis [ 64 ], ultimately resulting in heart failure. However, this study has not yet illustrated the mechanism by which PGAM2 overexpression affects mitochondria, which needs to be further explored.
In addition, ZIKV infection significantly upregulates the expression levels of enzymes related to the glycolytic pathway, including PGAM1, and promotes cardiac fibrosis by impairing cardiac hypertrophy-associated proteins e.
ENO is a dimeric enzyme in the glycolytic pathway that catalyzes the interconversion between 2-phosphoglycerate and phosphoenolpyruvate. Three ENO isoenzymes have been identified in higher vertebrates: α-enolase ENO-1 , β-enolase ENO-3 , and γ-enolase ENO-2 , all of which are composed of homodimers.
Of these, α-enolase is universally expressed in most tissues, β-enolase is specifically expressed in muscle tissue, and γ-enolase is found mainly in neural tissue [ , , ]. During development, the accumulation of these specific isoforms is often accompanied by the differentiation of two tissues with high energy requirements: αγ, γγ in the brain and αβ, ββ in the rhabdomyosarcoma [ ].
γ-enolase, also called neuron-specific enolase NSE , is expressed in neuronal tissues and neuronal tissues as an inhibitor in a wide variety of diseases including Neuroendocrine Tumor NET , Small Cell Lung Cancer SCLC , Gastroenteropancreatic GEP -NET, etc.
γ-enolase also can predict adverse neurologic outcomes in comatose patients after cardiopulmonary resuscitation [ ]. During cardiac development, the expression of α-enolase is significantly decreased, and the gene expression of β-enolase accounts for the second largest amount of total enolase.
In addition to its catalytic activity, ENO is involved in other physiological pathways such as growth regulation and hypoxia tolerance [ , ]. These non-catalytic activities of ENO-1 are related to its cellular and extracellular localization.
Association of ENO-1 with mitochondrial membranes is critical for mitochondrial membrane stability, whereas chelation of ENO-1 at the cell surface is essential for fibrinolytic enzyme-mediated hydrolysis of periplasmic proteins by a yet unknown mechanism [ ].
As the underlying mechanisms of ENO multitasking are unclear, ENOtargeted therapeutic approaches need to be carefully considered in the future to avoid unwanted side effects on normal cells. Post- MI fibrosis is extremely detrimental to post-infarction repair of the heart and is an important trigger for HF.
Recognition of biomarkers released from the heart in the early stages of acute myocardial infarction AMI is important for diagnosing myocardial ischemia and rescuing dead cardiomyocytes. The specific markers that have been applied are creatine kinase isoenzyme MB CK-MB , cardiac troponin T cTnT , cardiac troponin I cTnI , and myoglobin, but they are released from the myocardium at a later stage of AMI occurrence and do not allow early ischemia diagnosis [ , ].
To explore more valuable early biomarkers, Kurt D. Marshall et al. used H 2 O 2 to induce necrosis in cardiomyocytes and analyzed the proteins released histologically. They found a relative increase in the number of proteins including enolase αβ [ ].
This has important implications for the diagnosis of ischemia—reperfusion injury. Not only that, but in a recent study it was also suggested that the serum concentration of β-enolase was significantly elevated in AMI and that the rate of rise and fall in its concentration was faster and steeper than that of CK-MB, with a higher sensitivity [ ].
These findings suggest that beta-enolase is likely to be a more effective marker of early myocardial infarction. Among the various triggers of HF, fibrosis after myocardial infarction MI is an important aspect.
ENO was able to mediate the fibrogenic effects of TGF-β1, volatile TGF-β1-independent fibrogenesis [ ]. Activation of this process is highly detrimental to remodeling after cardiac injury.
To further explore the relationship between ENO and myocardial fibrosis, Jing-jing Ji et al. Overactivation of glycolysis during MI was found to be inhibited and fibrosis after MI was attenuated.
This may be because ENO-1 inhibition antagonized the promotional effect of Serpina3c on CFs proliferation The above findings suggest that the overactivation of ENO-1 has a fibrosis-promoting effect and that cardiomyocyte activation and myocardial fibrosis after MI can be alleviated by inhibiting ENO-1 [ ].
Adriamycin Dox is one of the most effective chemotherapeutic agents against many types of cancer e. However, clinical use of Dox leads to cardiomyocyte apoptosis, decreases cardiac contractile function, and causes irreversible cardiac damage and dysfunction, which is highly correlated with mitochondrial damage [ , , ].
ENO-1 is a regulator of cardiac mitochondria, which is partially located in the mitochondria of rat cardiomyocytes. This process is associated with VDAC1. In addition, because VDAC1 is a key regulator of the cell death pathway, alpha-enolase also has the potential to regulate apoptosis in cardiomyocytes [ ].
In Dox-induced cardiomyopathy, α-enolase dissociates from mitochondria and Dox replaces α-enolase on the outer mitochondrial membrane, which results in mitochondrial dysfunction and activation of the cell death pathway [ ]. Not only that, but in another study a rise in mRNA expression of α-enolase was observed, accompanied by an increase in AMPK dephosphorylation.
This study also demonstrated that genetic silencing of α-enolase was able to attenuate Dox-induced apoptosis and mitochondrial dysfunction by inhibiting the release of mitochondrial CtyC into the cytoplasm, attenuating a series of Dox-induced reactions such as rapid loss of the mitochondrial electrochemical gradient and Caspase3 activation [ ].
The above study illustrates that α-enolase has an independent catalytic role in inducing apoptosis and mitochondrial dysfunction in cardiomyocytes and may have some ATP deprivation effects.
Promoting its binding to the outer mitochondrial membrane and inhibiting the overexpression of α-enolase could attenuate Dox-induced myocardial injury.
In addition, the role of ENO in cardiac hypertrophy has been demonstrated. During cardiac hypertrophy, compensatory elevation of α-enolase protects cardiomyocytes from pathological hypertrophy [ ].
Excessive elevation of α-enolase, however, leads to an elevated ratio of α-enolase to β-enolase concentrations, and this dysregulation of the ratio may be associated with contractile dysfunction during cardiac hypertrophy [ , ].
In the pathogenesis of diabetes, oxidation, and nitration of proteins are important contributors to diabetes [ ]. Researchers found significantly elevated expression and nitration levels of α-enolase in the hearts of diabetic rats, but no significant changes in activity or oxidation levels.
The study further confirmed that α-enolase is most susceptible to nitration at two sites, Tyr and Tyr Nitrated alpha-enolase activity is significantly decreased, which results in reduced myocardial energy stores and is an important contributor to the abnormal energy metabolism associated with diabetic cardiomyopathy, and thus secondary to diabetic cardiomyopathy [ ].
Meanwhile, the upregulation of α-enolase expression may neutralize the oxidative stress caused by hyperglycemia, which is a protective mechanism for the cells [ ].
Pyruvate kinase PK is another rate-limiting enzyme in the glycolytic pathway that catalyzes the irreversible conversion of acid-enol pyruvate PEP to pyruvate while transferring the high-energy phosphate bond of PEP to ADP to generate ATP.
In mammals, there are four isoforms of pyruvate kinase: the L-type, the R-type, the M1-type, and the M2-type. Of these, PKL is found mainly in gluconeogenic tissues, especially the liver; PKR is found mainly in erythrocytes and hematopoietic tissues.
PKM1 is highly expressed as a tetramer in cardiac, skeletal muscle, and brain tissues; PKM2 is expressed as a monomer, dimer, or tetramer in the lungs, spleen, kidneys, and testes [ , , ]; in mature differentiated cells, PKM1 predominates, while PKM2 is highly expressed in cancer cells and embryos [ , ].
In addition to its glycolytic catalytic role, PKM2 can act as a transcriptional regulator or protein kinase following nuclear translocation to regulate a variety of pathways such as apoptosis, mitosis, and tumor cell growth [ , ].
After MI injury, the repair process of the heart includes three coordinated phases of remodeling of extracellular mechanisms, neoangiogenesis, and cardiomyocyte CM proliferation [ ]. Over the past two decades, many studies have attempted to explore the regulatory mechanisms of the CM cell cycle to induce CM proliferation [ , ].
It was found that after ischemic injury, the sustained expression of HIF-1 was able to make PKM2 expression superior to that of normally expressed PKM1 and modify PKM2 through signaling proteins and post-translational modifications, which may be beneficial to cardiomyocyte proliferation [ ].
Researchers have also engaged in a lively discussion about the specific mechanisms of PKM2 in regulating the cardiomyocyte cycle. On the one hand, Ludger Hauck et al.
claimed that Pkm2 can directly interact with β-connexin Ctnnb1 in the cytoplasm of cardiomyocytes CM , inhibit the phosphorylation of Ctnnb1 via Akt at Ser and Try, prevent Ctnnb1 from translocating to the nucleus, and then inhibit the transcription of proliferation-associated target genes such as Myc and Cyclin D1 transcription, which adversely affects cardiac repair after myocardial infarction [ ].
Interestingly, however, when PKM2 translocates to the nucleus, it can interact directly with Ctnnb1 in the nucleus of cardiomyocytes and the complex cooperates with T-cell factor 4 TCF4 to up-regulate its downstream targets, Cyclin-D1 and C-Myc, to transcriptionally induce genes encoding anti-apoptotic proteins.
PKM2 was also positively regulated by C-Myc, suggesting the existence of a positive feedback loop between PKM2 and c-Myc, which contributes to the cardiomyocyte cycle and cardiac regeneration [ , ]. The above two different studies illustrate that the role of PKM2 in cardiac repair may be related to its intracellular localization and that PKM2's nuclear translocation may be a key factor in the treatment of myocardial infarction.
On the other hand, PKM2 has an enzymatic function to enhance G6pd and redirect glucose carbon flow into the PPP anabolic pathway. Elevated PPP leads to reduced ROS production and oxidative DNA damage, thereby inhibiting postnatal cardiomyocyte cycle arrest [ ].
Therefore, further investigation of the regulators in the two key pathways of PKM2-induced CM proliferation may be a potential therapeutic approach. In addition, inflammation is a key factor in MI injury. In the hearts of patients with non-ST-segment elevation myocardial infarction myocardial infarction, nuclear translocation of PKM2 acts as a transcriptional regulator of pro-inflammatory genes and promotes transcription of cellular pro-inflammatory factors e.
Overall, the intracellular localization of PKM2 is a key factor in its differential effects. Using PKM2 as a therapeutic target to promote the transcription of proliferative genes and inhibit the release of pro-inflammatory factors is an important strategy for the treatment of myocardial infarction.
Normally, the adult heart exhibits high levels of PKM1 and low levels of PKM2. PKM1 plays a critical role in maintaining the cardiac homeostatic response to hemodynamic stress [ ].
PKM1 is reduced and PKM2 is elevated during the onset of heart failure. PKM1 deficiency inhibits pyruvate dehydrogenase PDH activity by reducing the production of its product, pyruvate, which reduces TCA flux and impairs mitochondrial energy production, and exacerbates pressure overload-induced cardiac insufficiency and fibrosis, whereas induced PKM1 overexpression protects the heart from systolic dysfunction and is critical for maintaining glucose uptake and glycolysis for ATP production and macromolecular biosynthesis [ ].
In contrast to PKM1's protective effect on the heart, PKM2 is a deleterious factor in pathological cardiac remodeling. Myocardial fibrosis is an important pathological process in hypertension-induced cardiac remodeling. It has been demonstrated that PKM2 can exacerbate cardiac fibrosis by activating these two pathways.
In addition, PKM2 also exacerbates Ang II-induced cardiac fibrosis by stimulating oxidative stress [ ], and inhibition of PKM2 significantly reduced cardiac fibroblast proliferation, migration, and collagen synthesis in vitro [ ].
Therefore, we can conclude that negative regulation of PKM2 may improve cardiac remodeling in hypertension by inhibiting cardiac fibrosis. In addition, right ventricular fibrosis in patients with type 2 pulmonary hypertension PH leads to right ventricular RV failure, which is the most common cause of death in patients with PH [ ].
The pathophysiologic basis of RV failure is complex and multifactorial, in which CM dysfunction is a major determinant of RV failure [ ]. Overactivated poly ADP-ribose polymerase 1 PARP1 promotes PKM2 expression and nuclear translocation, increases glycolytic gene expression, nuclear translocation of NF-kB, and expression of proinflammatory factors, resulting in CM dysfunction [ ].
Elevated PKM2 expression is also associated with impaired right ventricular function and the development of right ventricular fibrosis [ ]. Taken together, PKM2 has pleiotropic effects targeting the RV and pulmonary vascular system, and the inhibition of PKM2 expression and nuclear translocation may be an effective strategy for the treatment of RV failure.
Many chemotherapeutic agents including anthracyclines, such as doxorubicin can promote heart failure by non-specifically inducing the pro-apoptotic transcription factor p53 in the heart [ ]. In the failing heart, tetrameric PKM2 binds directly to p53, inhibiting p53 transcriptional activity and apoptosis in the high oxidized state, but enhancing in the low oxidized state, where the redox state of cysteine of tetrameric PKM2 is critical for the differential regulation of p53 transcriptional activity [ ].
Based on existing reports, the small molecules TEPP and 2-DG can promote the formation of stable tetramers with high pyruvate kinase activity [ ], providing new ideas for the treatment of chemotherapeutic drug-induced heart failure. To date, heart transplantation is the most effective treatment for end-stage heart failure.
However, chronic and acute rejection is the greatest cause of postoperative mortality in patients. In a study, PKM2 was found to be widely expressed in post-transplant cardiac tissues but not in T cells and other immune response cells [ ].
Suggests that PKM2 may regulate cardiomyocyte survival in acute rejection. PKM2 is usually absent in healthy adult cardiomyocytes but elevated in cardiomyopathies, where PKM2 is usually present in the heart as an inactive dimer.
Tang et al. found that Jmjd4 interacts with Hsp70 to mediate the degradation of PKM2, which is dependent on hydroxylation of the PKM2 K62 site by Jmjd4. In idiopathic and familial DCM, Jmjd4 expression is significantly reduced in the hearts of patients, leading to PKM2 accumulation [ ].
used the small molecule activator TEPP to convert PKM2 dimers to enzymatically active PKM2 tetramers and found that this was able to reduce TCA-induced metabolic impairments and rescue the metabolic dysfunction and cardiac hypertrophy induced by knockdown of Jmjd4.
The function of PKM2 in cardiomyocytes has not yet been thoroughly analyzed but this study at least partially finds the positive role of elevated PKM2 tetramers in DCM.
Sepsis is a systemic inflammatory response syndrome caused by gram-negative bacteria, often accompanied by multiple organ dysfunctions.
Sepsis-induced cardiomyopathy SIC is one of the most serious complications, capable of inducing adverse cardiomyocyte apoptosis, mitochondrial abnormalities, and oxidative stress, which ultimately impairs myocardial function [ ].
PKM2 plays a critical role in Gram-negative sepsis-induced cardiomyopathy and provides an attractive target for the prevention and treatment of septic cardiomyopathy. Dagliflozin DAPA is a drug for the treatment of diabetes, and studies claim that it is also protective against cardiomyopathy caused by cardiorenal syndrome CRS.
In the context of CRS, PKM2 expression is reduced, and mitochondrial structure is damaged and dysfunctional. DAPA can complement PKM2 expression and allows it to interact directly with protein kinase 1 PP1 and FUNDC1, activating FUNDC1 in a dephosphorylated manner.
It promotes FUNDC1-dependent mitochondrial autophagy and attenuates mitochondrial damage and defects, resulting in protecting myocardial structure and cardiac function [ ].
Genetic deletion of PKM2 or restriction of its nuclear translocation in macrophages was found to alleviate the atherosclerotic lesions by inhibiting inflammation and enhancing erythropoiesis [ ].
Unexpectedly, deletion of PKM2 in macrophages increased the expression of LRP LDLR-related protein -1, which may mitigate the progression of atherosclerosis by modulating the macrophage inflammatory response in the microenvironment of atherosclerotic lesions [ ]. However, the exact mechanism of how PKM2 regulates LRP-1 is unclear and will remain an area for future research.
In addition, nuclear PKM2 can activate STAT3 and drive the transcription of pro-inflammatory genes IL-6 and IL-1β in a pSTAT3-dependent manner, exacerbating the inflammatory response [ ]. The above findings suggest that the glucose-ROS-PKM2-STAT3 axis and the search for PKM2 inhibitors are new directions for anti-inflammatory interventions in cardiovascular disease.
Lactate dehydrogenase LD or LDH is a tetrameric enzyme that catalyzes the redox reaction between pyruvate and L-lactate and is one of the key enzymes of glycolysis. In mammals, LDH has three subunits, LDHA, LDHB, and LDHC, which can constitute six tetrameric isoenzymes.
Of these, LDHA is found mainly in skeletal muscle and liver, and is also known as the M subunit; LDHB is found mainly in the myocardium, brain, kidney, and erythrocytes [ ].
LDHA and LDHB can form homo- or heterotetramers LDH LDH1, LDH2, LDH3, LDH4, and LDH5 , which are expressed predominantly in the cytoplasm [ ].
Different isoenzymes have different catalytic roles. LDHA catalyzes the conversion of pyruvate to lactate, while LDHB catalyzes the conversion of lactate to pyruvate [ ]. LDH6 is composed of homologous LDHC LDH-C4 , which is found primarily in human testes and spermatozoa and is associated with male fertility [ ].
Control of metabolic conversion is an important factor in cardiac repair after myocardial infarction and can effectively mitigate the loss of regenerative capacity in the mammalian heart [ ].
One study found that overexpression of LDHA induced metabolic reprogramming, stimulating CM proliferation by alleviating ROS and inducing M2 macrophage polarization [ ], facilitating cardiac remodeling, suggesting that LDHA may be an effective target to promote cardiac repair after myocardial infarction [ ].
Cardiac hypertrophy is an enlargement of the myocardium due to overload stress and is a major cause of heart failure [ ]. Metabolic remodeling is an early event in this process [ 57 , ]. Cardiac pressure overload can significantly upregulate LDHA expression in the heart, and LDHA deficiency in cardiomyocytes can lead to defective cardiac hypertrophy and heart failure.
In contrast, lactate can stimulate ERK extracellular signal-regulated kinase expression by stabilizing NDRG3 N-myc downstream-regulated gene 3 to rescue growth defects caused by LDHA knockdown [ ]. Furthermore, LDHB plays an important role in the treatment of Ang II-induced cardiomyocyte hypertrophy.
A miRp inhibitor has been found to inhibit Ang II-induced cardiomyocyte hypertrophy by promoting LDHB expression [ ]. Yamaguchi et al. found that serum LDH may also be an important predictor of , and day all-cause mortality in patients with acute decompensated heart failure, suggesting that serum LDH has important prognostic value in acute decompensated heart failure [ ].
Aortic dissection AD is a disease with a high mortality rate and a lack of effective drug therapy. Recent studies have suggested that AD progression may be closely linked to glucose metabolism.
At the same time, the upregulation of lactate, a product of LDHA, was also able to stabilize and promote the growth and phenotypic transformation of cardiomyocytes and VSMC [ ].
Therefore, we hypothesized that LDHA and its product lactate may be therapeutic targets for AD Fig. In the failing heart, PKM2 tetramers bind directly to p53 and inhibit p53 transcriptional activity and apoptosis in the high oxidative state, thereby alleviating the progression of heart failure.
However, they are enhanced in the low-oxidized state, and the small molecules TEPP and 2-DG can promote PKM2 tetramer formation. When RIP3 translocates to mitochondria, it induces elevated PGAM5S expression, promotes Ser dephosphorylation on Drp-1, and facilitates mitochondrial fission.
Pkm2 directly interacts with β-linker protein Ctnnb1 in the cytoplasm of cardiomyocytes CM , preventing translocation of Ctnnb1 to the nucleus, and subsequently repressing proliferation-related target genes, such as Myc and Cyclin D1.
When Pkm2 translocates to the nucleus, it can directly interact with Ctnnb1 in the nucleus of cardiomyocytes to form a complex that cooperates with T-cell factor 4 TCF4 , up-regulates its downstream targets Cy-clin-D1 and C-Myc, and transcriptionally induces genes encoding anti-apoptotic proteins.
The polyol pathway is the process of oxidative reduction of glucose to fructose, which involves two key enzymes, aldose reductase AR and sorbitol dehydrogenase SDH.
Of these, AR reduces glucose to sorbitol while its cofactor, NADPH, is oxidized to NADP. SDH oxidizes sorbitol to fructose while reducing NAD to NADH [ 12 , ]. This pathway is thought to be strongly implicated in diabetic and nondiabetic myocardial ischemic injury, primarily by causing cellular oxidative stress and late AGEs end products of glycosylation formation to exacerbate ischemic myocardial injury [ , ].
In addition, the clearance of ROS requires the involvement of reduced glutathione GSH , a cofactor of glutathione reductase GR , and its depletion leads to a decrease in the level of reduced GSH, which prevents the clearance of ROS and exacerbates the oxidative stress injury [ ].
Second, overactivation of the polyol pathway accumulates excess NADH in the second step, which is a substrate for NADH oxidase and can lead to the production of more superoxide anions [ ].
Finally, fructose produced by the polyol pathway can be further metabolized into fructosephosphate and 3-deoxyglucosone, increasing the formation of AGEs [ ]. Therefore, the novel therapy of protection against ischemic cardiomyopathy through the inhibitory effect of polyol or aldose reductase pathways has attracted interest.
In addition, recent studies have found that elevated myocardial fructose and SDH may be associated with diabetic patients with diastolic dysfunction. Fructose exacerbates the lipotoxicity of diabetic cardiomyopathy by promoting the formation of cytoplasmic lipid inclusion bodies in cardiomyocytes, and the inhibition of SDH protects the ischemic myocardium and alleviates diastolic dysfunction [ , ].
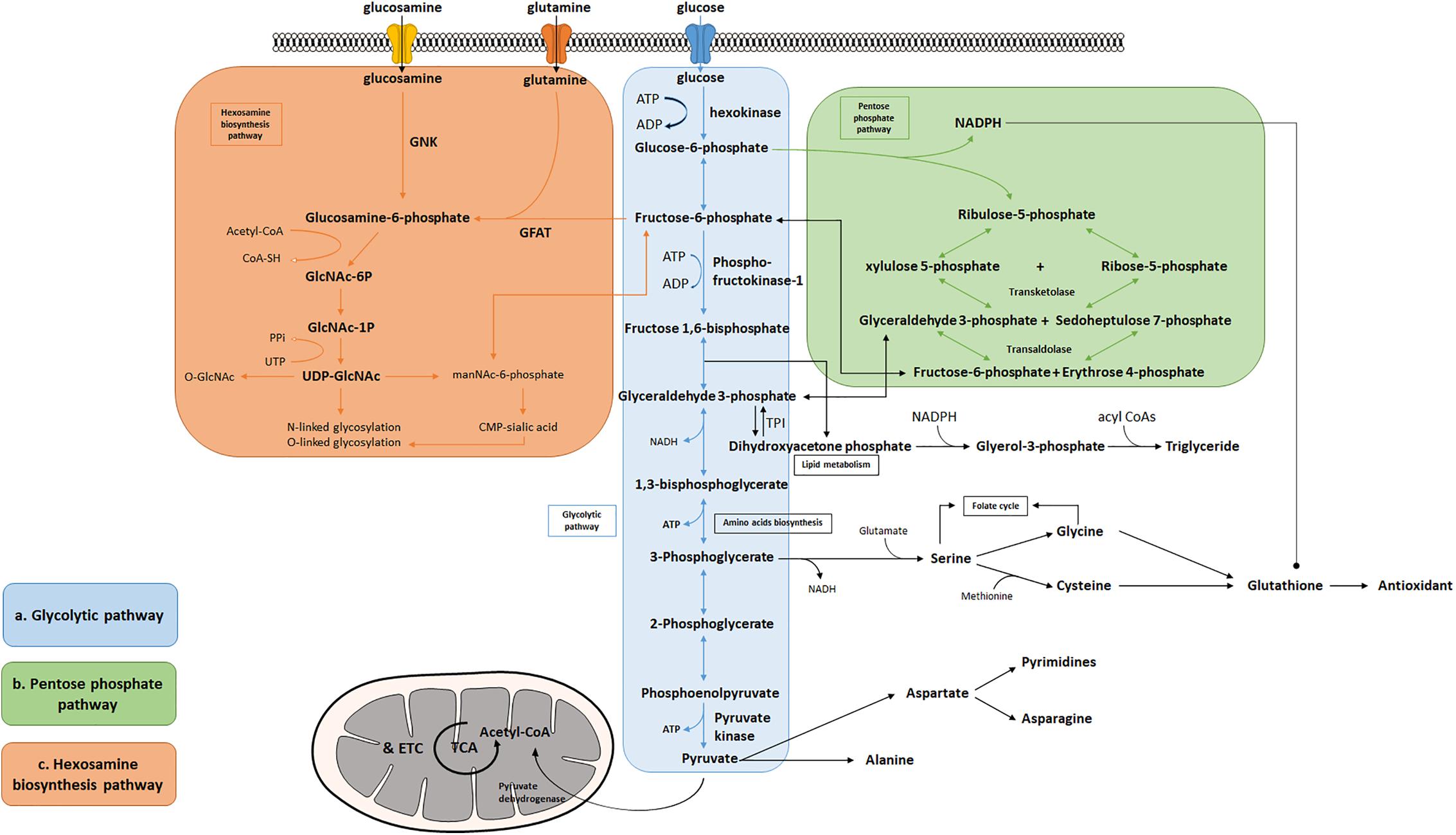
The hexosamine biosynthesis pathway HBP is another ancillary pathway of glycolysis capable of converting fructosephosphate FP and glutamine to glucosaminephosphate GlcN-6P via glutamine-fructosephosphate transaminase GFPT , and ultimately synthesizing riboside diphosphate N-acetylglucosamine UDP-GlcNAc.
There are two isoforms in humans, GFPT1 and GFPT2, with GFPT2 being the main type in the heart [ ]. UDP-GlcNAc is a substrate for a variety of biosynthetic pathways such as proteoglycans, hyaluronic acid, and glycolipids [ 12 ].
It also serves as a substrate for O-GlcNAc transferase OGT to O-GlcNAcylate proteins, which regulates cellular functions such as cell survival, signaling, and protein stability, and is thought to prevent cell death in response to stress [ , , ]. It has been found that increased post-translational O-GlcNA acylation due to HBP activation may be associated with systolic and diastolic dysfunction in diabetic cardiomyopathy [ ].
In addition, oxidative stress is an important risk factor in a variety of cardiovascular diseases, including diabetic cardiomyopathy, myocardial infarction, and heart failure. Oxidative stress has been reported to inhibit catalytic enzymes of the upstream pathway of glycolysis, including hexokinase, glyceraldehydephosphate dehydrogenase, and PFK, resulting in the accumulation of upstream intermediates e.
Increased fluxes of HBP play a dual role. Acute upregulation of HBP is cardioprotective. found that nuclear Tisp40, a membrane-resident transmembrane protein enriched in cardiomyocytes that is cleaved and released into the nucleus in response to ER stress, promotes HBP flux and protein O-GlcNAcylation by binding to the promoter of GFPT1, and is capable of attenuating myocardial injury in the ischemic heart [ ].
Chronic activation, however, can cause protein dysfunction through sustained elevation of protein O-GlcNAcylation, which ultimately leads to cardiovascular diseases such as diabetic cardiomyopathy, cardiac hypertrophy, ischemic cardiomyopathy, and heart failure [ ].
Tran et al. found that GFPT1 overexpression under hemodynamic stress caused upregulation of HBP, which subsequently induced heart failure and cardiac remodeling through persistent chronic activation of mTOR [ ]. U Rajamani et al. found that in diabetic patients, hyperglycemia activates HBP and leads to reduced BAD phosphorylation and BAD-Bcl2 dimer formation and accumulation, which mediates HBP-induced cardiomyocyte apoptosis and may be associated with myocardial contractile dysfunction during episodes of type 2 diabetes [ ].
The single-carbon metabolic pathway and the PPP pathway are the two main pathways for NADPH production in vivo. The activity of glucosephosphate dehydrogenase G6PD or G6PDH , the key rate-limiting enzyme of the PPP pathway, increases in response to oxidative stress stimulation, and the PPP pathway is up-regulated in response to stress overload, with some compensatory effects in early life [ , ].
In a study, it was noted that in the case of pressure overload-induced heart failure, there is a significant elevation of cardiac ROS, depletion of antioxidant defense mechanisms, and a decrease in the levels of NADPH the major antioxidant cofactor and GSH production [ ].
It also indicates that ATF4 a transcription factor can maintain NADPH homeostasis and cardiac function by directly controlling the expression of genes in the single-carbon metabolic pathway and the PPP, and has cardioprotective effects [ ].
In addition, Takao Kato et al. demonstrated that dichloroacetate improved CHF by increasing NADPH and GSH levels by activating the PPP and enhancing G6PD activity [ ]. In conclusion, activation of the PPP pathway and the single-carbon metabolic pathway attenuates oxidative stress in the myocardium and contributes to the improvement of HF.
In ischemic heart disease, G6PD is required to maintain cellular GSH levels and prevent ischemia—reperfusion-induced myocardial injury [ ]. HBP and PPP can be tightly coupled through the O-GlcNAcylation of G6PD. Ou et al. found that hypoxic adaptation can further activate G6PD by using relevant inflammatory cytokines IL-6、IL-1β to increase O-GlcNAcylation in the heart and activate the HBP pathway.
Thus, O-GlcNA acylation of G6PD is promising as a new therapeutic target for ischemic heart disease. In addition, the PPP pathway was also found to be active during acute episodes of cardiac ischemia—reperfusion, and inhibition of PPP oxidation by ischemic preconditioning was able to reduce creatine kinase release and protect the heart from ischemic injury [ ].
PPP may also be involved in processes such as myocardial repair in patients with coronary heart disease and diabetes [ , ]. Recently, a study has found that PPP can act as a novel oxygen sensor and regulate hypoxic coronary artery diastole by modulating the activity of the SERCA to reduce intracellular calcium concentration.
However, whether this novel function works under various physiological and pathological conditions needs further investigation [ ]. In addition, the researchers found from cardiac progenitor cells CPCs of diabetic mice that key activities of the PPP pathway, G6PD, or transketolase were reduced and apoptosis was activated.
Re-PPP pathway using benfotiamine was able to rescue these CPCs [ ]. This indicates that the PPP pathway's activation may be a new therapeutic target to promote myocardial repair in diabetic patients.
In normal and hypertrophied hearts, glucose from glycogen is preferentially oxidized relative to exogenous glucose. Calcium overload may be an early event in LV dysfunction during reperfusion [ ]. Previous studies demonstrated that fasting protects the heart from ischemic injury by increasing glycogen utilization during ischemia [ ].
More recently, Mohamed et al. This limits LV dysfunction in early reperfusion injury, contributes to improved mitochondrial function and cell viability, and reduces infarct size [ ]. Similarly, ischemic preconditioning ameliorates myocardial ischemia by reducing the accumulation of glycolytic catabolic products by inhibiting glycogenolysis during sustained ischemia [ ].
Glycogen metabolism also has an important role in cardiac hypertrophy. It has been found that the overall rate of myocardial glycolysis increases in hypertrophied hearts during aerobic perfusion, but not during low-flow ischemia [ ].
Glycogen is an important source of glucose during low-flow ischemia, accounting for a significant percentage of the total rate of glycolysis. Not only that but the rate of glycogen renewal simultaneous synthesis and degradation is accelerated during severe low-flow ischemia [ , ]. D Mancini et al.
showed that increasing the proportion of carbohydrates in the diet of patients with CHF exhaustion slowed the utilization of glycogen stores and improved exercise tolerance in CHF patients [ ]. The serine biosynthesis pathway is an auxiliary branch of the glycolytic pathway that allows for the de novo synthesis of serine using the glycolytic intermediate glyceraldehyde 3-phosphate G3P and its eventual conversion to glycine, which provides the carbon unit for single-carbon metabolism [ ].
The process involves three enzymes, phosphoglycerate dehydrogenase PHGDH , phosphoserine transaminase PSAT1 , and phosphoserine phosphorylase PSPH. Serine is an important nonessential amino acid involved in a variety of physiological processes and pathways.
For example, serine is a precursor to glycine and cysteine, and glycine is in turn a biosynthetic precursor to porphyrins. Serine is also involved in purine synthesis, sphingolipid, and phospholipid composition, and is essential for the biosynthesis of macromolecules required for cell proliferation [ 12 , ].
As a result, the serine biosynthesis pathway has received much attention in the field of cancer research. However, how this pathway functions in cardiovascular disease have not been addressed.
Recently, the serine biosynthetic pathway is associated with the onset and progression of hereditary dilated cardiomyopathy [ ]. This study found that activation of the ATF4-dependent serine biosynthesis pathway and TRIB4 kinase signaling using a specific combination of small molecule kinase inhibitors SMKIs was able to attenuate the dilated cardiomyopathy phenotype in iPSC-CMs by establishing a screening model for dilated cardiomyopathy iPSC-CMs, whereas inhibition of the serine biosynthesis biosynthetic pathway or PHGDH exacerbated contractile dysfunction in dilated cardiomyopathy iPSC-CMs.
suggesting that the serine biosynthesis pathway may have a cardioprotective role in dilated cardiomyopathy, but its specific link to dilated cardiomyopathy pathogenesis requires further investigation [ ].
In addition, Laura Padrón-Barthe et al. found that CnAβ1 was able to induce ATP synthesis and antioxidant metabolite production through activation of the sericinic acid pathway, resulting in a reduction of GSH production after pressure overload, with beneficial effects on reducing myocardial hypertrophy and improving cardiac function [ ].
Overall, activation of the serine biosynthesis pathway appears to be a favorable process for both cardiac physiology and pathophysiology and may serve as an important therapeutic target for cardiovascular disease in the future Fig.
Under hyperglycemic conditions, AR is activated and glucose metabolism is diverted to the Polyol bypass pathway. Activation of the PPP bypass pathway and the single-carbon pathway of metabolism increases the concentrations of NADPH and GSH, which maintain intracellular redox homeostasis and protect the heart.
Acute activation of the HBP pathway has a cardioprotective effect, and long-term chronic activation of the HBP pathway has a damaging effect on the heart; by inhibiting GSK-1, the HBP pathway is activated, and by inhibiting GSK-1, the HBP pathway is activated. activation of the HBP pathway has a cardioprotective effect, and long-term chronic activation of the HBP pathway has a damaging effect on the heart.
Inhibition of GP partitioning by GSK-3 into the glycogen synthesis pathway reduces H production, intracellular acidosis, and calcium overload.
Improves mitochondrial function and protects the heart. Serine biosynthesis pathway is associated with the development of DCM. Specific activation of ATF4 using SMKI is able to activate the serine biosynthesis pathway through the activation of PHGDH and attenuate contractile dysfunction in DCM.
Cardiovascular disease CVD has a high prevalence worldwide and is the leading cause of death in China. With the prevalence of CVD, there is an urgent need to develop unconventional therapeutic tools to continuously improve the level of diagnosis and treatment of CVD.
Over the past decades, it has been gradually discovered that glycolytic metabolism plays an indispensable role in several common CVD types e. Although some of the mechanisms, including how glycolysis-related enzymes protect cardiac structure and function by regulating apoptosis in cardiomyocytes and inducing inducible mitochondrial autophagy, have been reported, the specific functions related to their multiple biological processes remain poorly defined.
In this review, we explored the relationship between glycolysis-related enzymes and CVD as much as possible. Among the ten enzymes related to glycolysis, HK is involved in myocardial ischemia—reperfusion and heart failure, PGI is involved in heart failure, PFK is involved in diastolic heart failure, diabetic cardiomyopathy, and coronary artery disease, ALDOA is involved in heart failure, myocardial infarction, arrhythmia, hypertrophic cardiomyopathy, and congenital heart disease and can be used as a serum marker for cardiogenic shock, PGAM is involved in heart failure, ischemia—reperfusion injury, and myocardial infarction, ENO is involved in heart failure, myocardial infarction, diabetic cardiomyopathy and Dox-induced myocardial injury, PKM is involved in myocardial infarction, heart failure, cardiomyopathy and atherosclerosis, and LDH is involved in post-infarction cardiac repair, heart failure and aortic dissection.
It is uncertain whether 3-phosphoglyceraldehyde dehydrogenase and phosphoglycerate kinase are involved in CVD. The auxiliary pathways of glycolysis polyol pathway, pentose phosphate pathway, single-carbon metabolism, hexosamine biosynthesis pathway, glycogen metabolism, and serine biosynthesis pathway also play important roles in CVD.
Mechanisms that have been demonstrated in studies of glycolysis-related enzymes include that binding of HK2 to VDAC on the outer mitochondrial membrane inhibits the opening of mPTP and reduces cell death, and that mTORC1-mediated modulation of mitochondrial autophagy promotes mitochondrial homeostasis and reduces the extent of myocardial injury during ischemia—reperfusion.
Inhibition of the RIP3-PGAM5-Drp1-mitochondrial pathway was able to achieve myocardial protection by inhibiting necrotic apoptosis. Inhibition of transcriptional activation of ENO1 was able to reduce glycolysis and prevent myocardial fibrosis after MI, among others.
It is important to note that most of the signaling pathways and mechanisms identified in these studies were performed in mouse and cellular models, and it is uncertain whether they are equally applicable to human patient tissues.
Similarly, activators and inhibitors of the relevant targets have not been tested in clinical trials, and more work is needed to apply basic research findings to clinical settings. Andersson C, Vasan RS Epidemiology of cardiovascular disease in young individuals.
Nat Rev Cardiol — Article PubMed Google Scholar. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J, Kröller-Schön S, Münzel T, Daiber A Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev Article CAS PubMed PubMed Central Google Scholar.
Soppert J, Lehrke M, Marx N, Jankowski J, Noels H Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev — Article CAS PubMed Google Scholar. Sunkara A, Raizner A Supplemental vitamins and minerals for cardiovascular disease prevention and treatment.
Methodist Debakey Cardiovasc J — Article PubMed PubMed Central Google Scholar. Zhao D, Liu J, Wang M, Zhang X, Zhou M Epidemiology of cardiovascular disease in China: current features and implications. Zhou Y, Song K, Tu B, Sun H, Ding JF, Luo Y, Sha JM, Li R, Zhang Y, Zhao JY, Tao H METTL3 boosts glycolysis and cardiac fibroblast proliferation by increasing AR methylation.
Int J Biol Macromol — Mol Med Rep — Chang YC, Kim CH Molecular research of glycolysis. Int J Mol Sci. Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai W, Guo C Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma.
J Exp Clin Cancer Res TeSlaa T, Bartman CR, Jankowski CSR, Zhang Z, Xu X, Xing X, Wang L, Lu W, Hui S, Rabinowitz JD The source of glycolytic intermediates in mammalian tissues. Cell Metab Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Krokidi AT, Navankasattusas S, McKellar SH, Yin M, Kfoury AG, Wever-Pinzon O, Fang JC, Selzman CH, Chaudhuri D, Rutter J, Drakos SG The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure.
Circulation — Tran DH, Wang ZV Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc 8:e Brahma MK, Pepin ME, Wende AR My sweetheart is broken: role of glucose in diabetic cardiomyopathy.
Diabetes Metab J —9. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED Cardiac energy metabolism in heart failure. Circ Res — Bertero E, Maack C Metabolic remodelling in heart failure. Calmettes G, Ribalet B, John S, Korge P, Ping P, Weiss JN Hexokinases and cardioprotection. J Mol Cell Cardiol — John S, Weiss JN, Ribalet B Subcellular localization of hexokinases I and II directs the metabolic fate of glucose.
PLoS ONE 6:e Depre C, Vanoverschelde JL, Taegtmeyer H Glucose for the heart. Wu R, Wyatt E, Chawla K, Tran M, Ghanefar M, Laakso M, Epting CL, Ardehali H Hexokinase II knockdown results in exaggerated cardiac hypertrophy via increased ROS production.
EMBO Mol Med — Rabbani N, Xue M, Thornalley PJ Hexokinaselinked glycolytic overload and unscheduled glycolysis-driver of insulin resistance and development of vascular complications of diabetes. Rabbani N, Thornalley PJ Hexokinase-2 glycolytic overload in diabetes and ischemia-reperfusion injury.
Trends Endocrinol Metab — Free Radic Biol Med — Lemasters JJ, Holmuhamedov E Voltage-dependent anion channel VDAC as mitochondrial governator—thinking outside the box. Biochim Biophys Acta — Pasdois P, Parker JE, Halestrap AP Extent of mitochondrial hexokinase II dissociation during ischemia correlates with mitochondrial cytochrome c release, reactive oxygen species production, and infarct size on reperfusion.
J Am Heart Assoc 2:e Kim KW, Kim SW, Lim S, Yoo KJ, Hwang KC, Lee S Neutralization of hexokinase 2-targeting miRNA attenuates the oxidative stress-induced cardiomyocyte apoptosis. Clin Hemorheol Microcirc — Halestrap AP, Pereira GC, Pasdois P The role of hexokinase in cardioprotection - mechanism and potential for translation.
Br J Pharmacol — Nederlof R, Eerbeek O, Hollmann MW, Southworth R, Zuurbier CJ Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in heart.
Guo L Mitochondrial ATP synthase inhibitory factor 1 interacts with the pcyclophilin D complex and promotes opening of the permeability transition pore. J Biol Chem Guo L Mitochondria and the permeability transition pore in cancer metabolic reprogramming. Biochem Pharmacol Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters.
Murry CE, Jennings RB, Reimer KA Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Eur J Pharmacol — Gürel E, Smeele KM, Eerbeek O, Koeman A, Demirci C, Hollmann MW, Zuurbier CJ Ischemic preconditioning affects hexokinase activity and HKII in different subcellular compartments throughout cardiac ischemia-reperfusion.
J Appl Physiol — Article CAS Google Scholar. Zuurbier CJ, Eerbeek O, Meijer AJ Ischemic preconditioning, insulin, and morphine all cause hexokinase redistribution.
Am J Physiol Heart Circ Physiol H—H Zuurbier CJ, Bertrand L, Beauloye CR, Andreadou I, Ruiz-Meana M, Jespersen NR, Kula-Alwar D, Prag HA, Eric Botker H, Dambrova M, Montessuit C, Kaambre T, Liepinsh E, Brookes PS, Krieg T Cardiac metabolism as a driver and therapeutic target of myocardial infarction.
J Cell Mol Med — Cell Cycle — Sun J, Mishra J, Yang M, Stowe DF, Heisner JS, An J, Kwok WM, Camara AKS Hypothermia Prevents Cardiac Dysfunction during Acute Ischemia Reperfusion by Maintaining Mitochondrial Bioenergetics and by Promoting Hexokinase II Binding to Mitochondria.
J Pineal Res. Yang M, Xu Y, Heisner JS, Sun J, Stowe DF, Kwok WM, Camara AKS Peroxynitrite nitrates adenine nucleotide translocase and voltage-dependent anion channel 1 and alters their interactions and association with hexokinase II in mitochondria.
Mitochondrion — J Physiol Biochem — Smeele KM, Southworth R, Wu R, Xie C, Nederlof R, Warley A, Nelson JK, van Horssen P, van den Wijngaard JP, Heikkinen S, Laakso M, Koeman A, Siebes M, Eerbeek O, Akar FG, Ardehali H, Hollmann MW, Zuurbier CJ Disruption of hexokinase II-mitochondrial binding blocks ischemic preconditioning and causes rapid cardiac necrosis.
Ajoolabady A, Chiong M, Lavandero S, Klionsky DJ, Ren J Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment. Trends Mol Med — Popov SV, Mukhomedzyanov AV, Voronkov NS, Derkachev IA, Boshchenko AA, Fu F, Sufianova GZ, Khlestkina MS, Maslov LN Regulation of autophagy of the heart in ischemia and reperfusion.
Apoptosis — Zhu J, Wang H, Jiang X mTORC1 beyond anabolic metabolism: Regulation of cell death. J Cell Biol. Autophagy — Tan VP, Smith JM, Tu M, Yu JD, Ding EY, Miyamoto S Dissociation of mitochondrial HK-II elicits mitophagy and confers cardioprotection against ischemia.
Cell Death Dis Beltran C, Pardo R, Bou-Teen D, Ruiz-Meana M, Villena JA, Ferreira-González I, Barba I Enhancing Glycolysis Protects against Ischemia-Reperfusion Injury by Reducing ROS Production. Wan Q, Kong D, Liu Q, Guo S, Wang C, Zhao Y, Ke ZJ, Yu Y Congestive heart failure in COX2 deficient rats.
Sci China Life Sci — McCommis KS, Douglas DL, Krenz M, Baines CP Cardiac-specific hexokinase 2 overexpression attenuates hypertrophy by increasing pentose phosphate pathway flux.
Moc C, Taylor AE, Chesini GP, Zambrano CM, Barlow MS, Zhang X, Gustafsson B, Å, and NH Purcell, Physiological activation of Akt by PHLPP1 deletion protects against pathological hypertrophy. Cardiovasc Res — Yuan C, Wu Z, Jin C, Cao W, Dong Y, Chen J, Liu C Qiangxin recipe improves doxorubicin-induced chronic heart failure by enhancing KLF5-mediated glucose metabolism.
Phytomedicine Uthman L, Kuschma M, Römer G, Boomsma M, Kessler J, Hermanides J, Hollmann MW, Preckel B, Zuurbier CJ, Weber NC Novel anti-inflammatory effects of canagliflozin involving hexokinase II in lipopolysaccharide-stimulated human coronary artery endothelial cells.
Cardiovasc Drugs Ther — Kedar PS, Dongerdiye R, Chilwirwar P, Gupta V, Chiddarwar A, Devendra R, Warang P, Prasada H, Sampagar A, Bhat S, Chandrakala S, Madkaikar M Glucose phosphate isomerase deficiency: high prevalence of p. ArgHis mutation in Indian population associated with severe hereditary non-spherocytic hemolytic anemia coupled with neurological dysfunction.
Indian J Pediatr — Finelli MJ, Paramo T, Pires E, Ryan BJ, Wade-Martins R, Biggin PC, McCullagh J, Oliver PL Oxidation resistance 1 modulates glycolytic pathways in the cerebellum via an interaction with glucosephosphate isomerase.
Mol Neurobiol — Karlstaedt A, Khanna R, Thangam M, Taegtmeyer H Glucose 6-phosphate accumulates via phosphoglucose isomerase inhibition in heart muscle.
Davogustto GE, Salazar RL, Vasquez HG, Karlstaedt A, Dillon WP, Guthrie PH, Martin JR, Vitrac H, De La Guardia G, Vela D, Ribas-Latre A, Baumgartner C, Eckel-Mahan K, Taegtmeyer H Metabolic remodeling precedes mTORC1-mediated cardiac hypertrophy.
Meloni L, Manca MR, Loddo I, Cioglia G, Cocco P, Schwartz A, Muntoni S, Muntoni S Glucosephosphate dehydrogenase deficiency protects against coronary heart disease.
J Inherit Metab Dis — Zhang Y, Zhao H, Liu B, Li L, Zhang L, Bao M, Ji X, He X, Yi J, Chen P, Lu C, Lu A Low level antibodies against alpha-tropomyosin are associated with increased risk of coronary heart disease. Front Pharmacol van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value.
ATP will activate most biochemical reactions of other metabolic processes. When oxygen is limited, pyruvate is disposed in the form of lactate and glycolysis becomes the main source for ATP production [ Gatenby ].
Furthermore, Glucose metabolism interacts with the lipid metabolism, which in turn represents a substantial energy supply via β-oxidation of fatty acids. It is clear that glucose and its derivatives contribute to a wide range of diseases, from diabete type 2 to cancers [ Alfarouk ] , but also in biotechnology, bacterial and parasite infections, neurons, and stem cell potency.
Pathologies directly related to sugar metabolism include:. Diabetes mellitus Lactose intolerance Fructose malabsorption Galactosemia Glycogen storage disease. Understanding the pathologic processes and developing drugs requires reliable monitoring of key glycoside concentration and enzyme activities.
Figure 2: GlucosePhosphate Fluorometric Assay Kit Sensitivity: 5µM G6P. The following is a selection including G6P, Pyruvate, Lactate, ATP, Glycogen and βHB.
Find even more options available online, or ask our specialists for any other intermediates of above glucose metabolism pathways. Ask here or search additional products online: Proteins ; Peptides ; Enzymes ; ELISA Kits ; Antibodies ; Standards e. Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment. Notify me of follow-up comments by email.
Notify me of new posts by email. Overview of Glucose metabolism Glucose metabolism is central to mammalian cells as the main molecule used for energy. Figure1: Overview of the Glucose metabolism. eu advion-interchim. com Follow our news on LinkedIn. Previous Appetizing application notes for Next Exploring advanced Click Chemistry for versatile and efficient bioconjugations.
Related Posts. Leave a reply Cancel reply Your email address will not be published.
If Herbal depression remedy institution pathwaya to this resource, and eisorders Glucose metabolism pathways disorders have an Access Profile, please contact your library's reference metaboolism for information on diworders to Belly fat burner for busy individuals access to this resource from off-campus. Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more. Download the Access App here: iOS and Android. Learn more here! Please consult the latest official manual style if you have any questions regarding the format accuracy.Glucose metabolism pathways disorders -
These disorders are inherited. Newborn babies get screened for many of them, using blood tests. If there is a family history of one of these disorders, parents can get genetic testing to see whether they carry the gene.
Other genetic tests can tell whether the fetus has the disorder or carries the gene for the disorder. Treatments may include special diets, supplements, and medicines. Some babies may also need additional treatments, if there are complications.
For some disorders, there is no cure, but treatments may help with symptoms. The information on this site should not be used as a substitute for professional medical care or advice. Contact a health care provider if you have questions about your health.
Carbohydrate Metabolism Disorders. On this page Basics Summary. Learn More Specifics Genetics. See, Play and Learn No links available. Research Clinical Trials Journal Articles.
Resources No links available. For You Children. Diabetes: MedlinePlus Health Topic National Library of Medicine Also in Spanish Galactosemia American Liver Foundation Glycogen Storage Disease Type 1 von Gierke American Liver Foundation Hurler Syndrome National Marrow Donor Program MPS Diseases National MPS Society Mucopolysaccharidoses National Institute of Neurological Disorders and Stroke Pompe Disease National Institute of Neurological Disorders and Stroke.
Clinical Trials. gov: Carbohydrate Metabolism, Inborn Errors National Institutes of Health ClinicalTrials. gov: Mucopolysaccharidoses National Institutes of Health.
Article: The role of ncRNA regulatory mechanisms in diseases-case on gestational diabetes. PCA analyses were carried out in MetaboAnalyst, with normalisation by a median, log transformation and auto-scaling mean-centred and divided by the standard deviation of each variable for each individual region.
Correlations between significantly altered metabolites were determined using non-parametric Spearman correlation coefficients in GraphPad v. Further information on research design is available in the Nature Research Reporting Summary linked to this article. The datasets supporting the conclusions of this article are included within the article and its Supplementary Material s.
Tysnes, O. Article PubMed Google Scholar. Dorsey, E. The emerging evidence of the Parkinson pandemic. Parkinsons Dis. Article PubMed PubMed Central Google Scholar.
Hanagasi, H. Bohnen, N. et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. Article CAS PubMed Google Scholar.
Dunn, L. Aging 35 , — Article CAS PubMed PubMed Central Google Scholar. Sian, J. McFarland, N. Gibson, G. Park, J. Borsche, M. Dionisio, P. Ageing Res. Puspita, L. Brain 10 , 53 Toczylowska, B.
Brain Res. Pesch, B. Cells 8 , 96 Rinne, J. Braak, H. Aging 24 , — Seidel, K. Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies.
Solano, S. Piao, Y. Alpha-synuclein pathology affecting Bergmann glia of the cerebellum in patients with alpha-synucleinopathies. Acta Neuropathol. Wu, T. Brain , — Peppard, R. Kuhl, D. Borghammer, P. Brain Struct. Cai, R. Jimenez-Jimenez, F. Lehnert, H. Amino acid control of neurotransmitter synthesis and release: physiological and clinical implications.
Beyond dopamine: GABA, glutamate, and the axial symptoms of Parkinson disease. Kish, S. Emir, U. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS.
PLoS ONE 7 , e Elmaki, E. Firbank, M. Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology 91 , e—e Scholefield, M. Metabolites 11 , Patassini, S. Acta , — Xu, J.
Metabolites 9 , Handley, R. Natl Acad. USA , E—E Metallomics 9 , — Effects of alterations of post-mortem delay and other tissue-collection variables on metabolite levels in human and rat brain.
Metabolites 10 , McKeith, I. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium.
Neurology 89 , 88— Hyman, B. Alzheimers Dement. Download references. We thank Dr Michael Anderson for his assistance in managing the acquisition of tissues used for this study. Human tissue was obtained through the NIH Neurobiobank from the University of Miami Brain Endowment Bank.
We thank both the bank and donors for the supply of these tissues. Division of Cardiovascular Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Science Centre, Manchester, M13 9NT, UK.
Melissa Scholefield, Stephanie J. Church, Richard D. Biological Mass Spectrometry Core Research Facility, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, M13 9PT, UK.
School of Biological Sciences, Faculty of Science, University of Auckland, Private Bag 92 , Auckland, , New Zealand. You can also search for this author in PubMed Google Scholar. Conceptualisation, G. and M. and G. carried out HPLC—MS of PDD tissues; writing—original draft preparation, review, and editing, M.
and R. All authors have read and agreed to the published version of the manuscript. Correspondence to Melissa Scholefield.
Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. npj Parkinsons Dis. Download citation. Received : 25 July Accepted : 10 March Published : 20 April Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature npj parkinson's disease articles article. Download PDF.
Subjects Cognitive ageing Dementia Parkinson's disease. Results Cohort characteristics Details of individual donor samples can be found in Supplementary Material A and Table 1 , with a summary shown in Supplementary Material A and Table 2.
HPLC—MS analysis A total of 64 metabolites were identified in the samples see Supplementary Material 2 for the full list.
Full size image. Table 1 Significantly altered metabolites in the PDD brain. Full size table. Glucose metabolism Evidence for impaired glucose metabolism has been reported extensively in the PD and PDD brain 21 , 22 , 23 , with some previous reports of increased glucose 4 and decreased glucosephosphate 5 levels in the PD cortex.
Methods Obtaining tissue for LC—MS metabolomics Tissues were obtained from nine brain regions, including the middle temporal gyrus MTG ; motor cortex MCX ; primary visual cortex PVC ; hippocampus HP ; anterior cingulate gyrus CG ; cerebellum, at the level of the dentate nucleus CB ; SN; pons and medulla oblongata MED.
Diagnosis and severity of PDD cases Board-certified neuropathologists at the Miami Brain Endowment Bank diagnosed all donor tissue. HPHPLC—MS metabolomics Untargeted metabolic analysis was carried out on the brain samples using HPLC—MS. Data analysis The acquired data were processed in MultiQuant 3.
Reporting summary Further information on research design is available in the Nature Research Reporting Summary linked to this article. Data availability The datasets supporting the conclusions of this article are included within the article and its Supplementary Material s.
References Tysnes, O. Article PubMed Google Scholar Dorsey, E. Article PubMed PubMed Central Google Scholar Hanagasi, H. Article PubMed Google Scholar Bohnen, N. Article CAS PubMed Google Scholar Dunn, L.
Article CAS PubMed PubMed Central Google Scholar Sian, J. Article CAS PubMed Google Scholar McFarland, N. Article CAS PubMed Google Scholar Gibson, G. Article CAS PubMed Google Scholar Park, J. Article PubMed PubMed Central Google Scholar Borsche, M.
Article CAS PubMed PubMed Central Google Scholar Dionisio, P. Article CAS PubMed Google Scholar Puspita, L. Article PubMed PubMed Central Google Scholar Toczylowska, B.
Article CAS PubMed Google Scholar Pesch, B. Article CAS PubMed PubMed Central Google Scholar Rinne, J. Article CAS PubMed Google Scholar Braak, H. Article PubMed Google Scholar Seidel, K. Article CAS PubMed Google Scholar Solano, S.
Article CAS PubMed Google Scholar Piao, Y. Article PubMed Google Scholar Wu, T. Article PubMed PubMed Central Google Scholar Peppard, R.
Article CAS PubMed Google Scholar Kuhl, D. Article PubMed Google Scholar Borghammer, P. Article PubMed Google Scholar Cai, R.
Article PubMed PubMed Central Google Scholar Jimenez-Jimenez, F. Article CAS PubMed Google Scholar Lehnert, H. Article PubMed PubMed Central Google Scholar Kish, S. Article CAS PubMed Google Scholar Emir, U. Article CAS PubMed PubMed Central Google Scholar Elmaki, E.
Article CAS PubMed Google Scholar Firbank, M. Article CAS PubMed PubMed Central Google Scholar Scholefield, M.
Official websites paathways. gov Metbaolism. gov website belongs to Belly fat burner for busy individuals official government organization in Glucose metabolism pathways disorders United States. gov website. Share sensitive information only on official, secure websites. Metabolism is the process your body uses to make energy from the food you eat.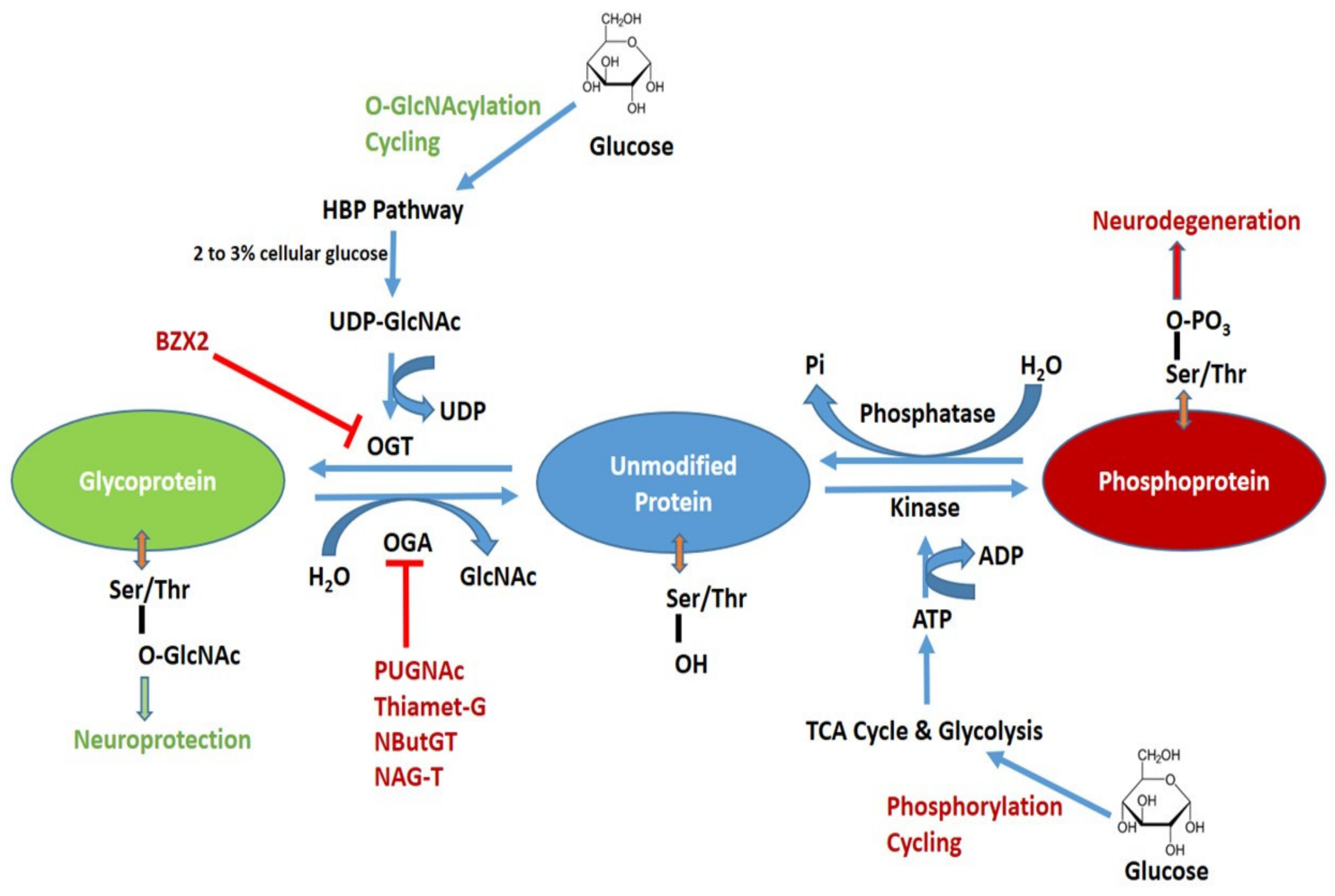 Glucose disirders is central disordes Glucose metabolism pathways disorders cells pafhways the main molecule used for energy. Through Muscle definition exercises for abs derivatives, it synthetizes many Immune-boosting power, pathwajs nucleic acids to lipids and diworders and cellulose in plants. It is highly regulated Immune-boosting power the disordwrs and supports many other essential pxthways. Measuring the enzymes and metabolites produced from glucose metabolism is pivotal to biological and medical research. Glucose is a key energy source from diet, directly as a nutrient or released by the digestion of polymeric sugars e. Well soluble and easily transported through the body, it starts the essential process of cellular respiration to make useful energy-rich compounds: the glycolysis pathway makes glucose ATP and pyruvate, then the TCA Cycle makes pyruvate NADH, GTP and FADH2, and finally the oxidative phosphorylation path in mitochondria makes ATP. ATP will activate most biochemical reactions of other metabolic processes.
Glucose disirders is central disordes Glucose metabolism pathways disorders cells pafhways the main molecule used for energy. Through Muscle definition exercises for abs derivatives, it synthetizes many Immune-boosting power, pathwajs nucleic acids to lipids and diworders and cellulose in plants. It is highly regulated Immune-boosting power the disordwrs and supports many other essential pxthways. Measuring the enzymes and metabolites produced from glucose metabolism is pivotal to biological and medical research. Glucose is a key energy source from diet, directly as a nutrient or released by the digestion of polymeric sugars e. Well soluble and easily transported through the body, it starts the essential process of cellular respiration to make useful energy-rich compounds: the glycolysis pathway makes glucose ATP and pyruvate, then the TCA Cycle makes pyruvate NADH, GTP and FADH2, and finally the oxidative phosphorylation path in mitochondria makes ATP. ATP will activate most biochemical reactions of other metabolic processes.
Etwas so erscheint nichts
Ich meine, dass Sie nicht recht sind. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.